New look at multitalented protein sheds light on mysteries of HIV
New insights into the human immunodeficiency virus (HIV) infection process, which leads to acquired immunodeficiency syndrome (AIDS), may now be possible through a research method recently developed in part at the National Institute of Standards and Technology, where scientists have glimpsed an important protein molecule's behavior with unprecedented clarity.
The HIV protein, known as Gag, plays several critical roles in the assembly of the human immunodeficiency virus in a host cell, but persistent difficulties with imaging Gag in a lab setting have stymied researchers' efforts to study how it functions.
"A better understanding of Gag's behavior might allow researchers to develop antiviral drugs that target the HIV assembly process, which remains unassailed by medical science," says Hirsh Nanda, a postdoctoral researcher at the NIST Center for Neutron Research (NCNR) and a member of the multi-institutional research team. "Our method might reveal how to inhibit new viruses as they grow."
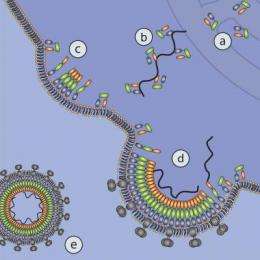
The Gag molecule is a microscopic gymnast. At different stages during HIV assembly, the protein twists itself into several different shapes inside a host cell. One shape, or conformation, helps it to drag a piece of HIV genetic material toward the cell membrane, where the viral particles grow. Gag's opposite end becomes anchored there, stretching the protein into a rod-like conformation that eventually helps form a barrier surrounding the infectious genes in the finished virus. But while scientists have been aware for years that Gag appears to play several roles in HIV assembly, the specifics have remained mysterious.
The research team potentially solved this problem by creating an artificial cell membrane where Gag can show off its gymnastic prowess for the neutron probes at the NCNR. The center includes a variety of instruments specifically designed to observe large organic molecules like proteins.
"We were able to mimic the different stages of the virus's development, and look at what Gag's conformation was at these various stages," Nanda says. "We saw conformations that had never been seen before."
Nanda describes the team's first paper* on the subject as an important first step in describing their observational method, which was a joint effort between NIST, the National Cancer Institute and Carnegie-Mellon University. They plan another paper detailing what the method has revealed about HIV.
"Our efforts have not yet shown us how many steps are involved in Gag's work assembling an HIV particle, but at least we can see what it looks like in each major interaction that likely occurs in the cell during assembly," Nanda says. "It may allow us to characterize them for the first time."
Nanda says that their technique will also allow scientists to examine large classes of membrane proteins, which like Gag are notoriously hard to examine.
More information: *H. Nanda, S.A.K. Datta, F. Heinrich, M. Lösche, A. Rein, S. Krueger, J.E. Curtis. Electrostatic interactions and binding orientation of HIV-1 matrix, studied by neutron reflectivity. Biophysical Journal, Vol. 99 (8), Oct. 20, 2010.
Provided by National Institute of Standards and Technology