Molecular fossil: Crystal structure shows how RNA, one of biology's oldest catalysts, is made
(PhysOrg.com) -- In today's world of sophisticated organisms proteins are the stars. They are the indispensible catalytic workhorses, carrying out the processes essential to life. But long, long ago ribonucleic acid (RNA) reigned supreme.
Now Northwestern University researchers have produced an atomic picture that shows how two of these very old molecules interact with each other. It is a rare glimpse of the transition from an ancient, RNA-based world to our present, protein-catalyst dominated world.
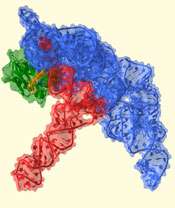
The scientists are the first to show the atomic details of how ribonuclease P (RNase P) recognizes, binds and cleaves transfer RNA (tRNA). They used the powerful X-rays produced by the Advanced Photon Source at Argonne National Laboratory to obtain images from crystals formed by these two RNA molecules. The result is a snapshot of one of the most complex models of a catalytic RNA and its target.
Details of the structure will be published Nov. 14 by the journal Nature.
"RNA is an ancient molecule, but it is pretty sophisticated," said Alfonso Mondragón, professor of molecular biosciences in the Weinberg College of Arts and Sciences. He led the research. "Our crystal structure shows that it has many of the properties we ascribe to modern molecules. RNA is a catalyst that has much of the versatility and complexity of modern-day proteins."
For billions of years and still to this day, the function of RNase P -- found in nearly all organisms, from bacteria to humans -- has been to cleave transfer tRNA. If the tRNA is not cleaved, it is not useful to the cell.
"We knew this important chemistry happened, that RNA acts as a catalyst, but we didn't know exactly how until now," Mondragón said. "We now have a better understanding of how RNA works."
RNase P is formed by a large RNA core plus a small protein, illustrating the evolutionary shift from an RNA world toward a protein-dominated world. The protein helps recognize the tRNA, but most of the recognition occurs through RNA-RNA interactions involving shape complementarity and also base pairing.
The structure shows that once RNase P recognizes tRNA, it docks and, assisted by metal ions, cuts one chemical bond. This matures the tRNA, producing a smaller RNA molecule that now can contribute to fundamental processes in the cell. The RNA-based enzyme does this over and over, cutting each tRNA in exactly the same place every time.
"The discovery nearly 30 years ago that RNA molecules can have a catalytic function raised the idea that maybe RNA was the first molecule," Mondragón said. "Our work reinforces this notion of the existence of an RNA world when life first began."
More information: The title of the Nature paper is "Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA."
Provided by Northwestern University