Pivoting hooks of graphene's chemical cousin could revolutionize work of electron microscopes
The single layer material Graphene was the subject of a Nobel prize this year but research led by a team of researchers at the University of Warwick has found molecular hooks on the surface of its close chemical cousin, Graphene Oxide, that will potentially provide massive benefits to researchers using transmission electron microscopes. They could even be used in building molecular scale mechanisms.
The research team, which includes Drs. Jeremy Sloan, Neil Wilson and PhD student Priyanka Pandey from the Department of Physics and Dr. Jon Rourke from the Department of Chemistry together with the groups of Drs. Kazu Suenaga and Zheng Liu from AIST in Japan and Drs. Ian Shannon and Laura Perkins in Birmingham were looking at the possibility of using Graphene as a base to mount single molecules for imaging by transmission electron microscopy. As Graphene forms an electron transparent sheet just one atom thick it would enable high precision, high contrast imaging of the molecules being studied as well as the study of any interactions they have with the supporting graphene.
While this idea is great in theory, Graphene is actually very difficult to create and manipulate in practice. The researchers therefore turned to Graphene's easier to handle cousin, Graphene Oxide. This choice turned out to be a spectacularly better material as they found extremely useful properties, in the form of ready-made molecular hooks that could make Graphene Oxide the support material of choice for future transmission electron microscopy of any molecule with oxygen on its surface.
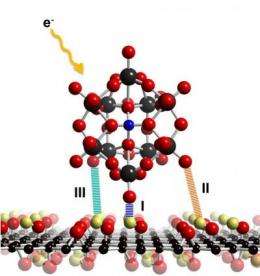
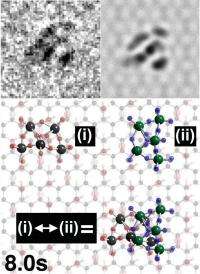
Graphene Oxide's name obscures the fact that it is actually a combination of carbon, oxygen and hydrogen. For the most part it still resembles the one atom thin sheet of pure Graphene, but it also has "functional groups" consisting of hydrogen paired with oxygen. These functional groups can bind strongly to molecules with external oxygens making them ideal tethers for researchers wishing to study them by transmission electron microscoscopy.
This feature alone will probably be enough to persuade many researchers to turn to Graphene Oxide as a support for the analysis of a range of molecules by transmission electron microscopy, but the researchers found yet another intriguing property of these handy hooks – the molecules attached to them move and pivot around them.
Dr Jeremy Sloan said: "Under the right conditions the functional groups not only provide molecular tethers that hold molecules in an exact spot they also allow the molecule to be spun in that position. This opens up a range of new opportunities for the analysis of such molecules but could also be a useful mechanism for anyone seeking to create molecular sized "machinery"."
More information: The research paper is entitled "Imaging the Structure, Symmetry, and Surface-Inhibited Rotation of Polyoxometalate Ions on Graphene Oxide" is published in Nano Letters and is by Dr Jeremy Sloan, Jonathan P. Rourke, Neil R. Wilson and Priyanka A. Pandey from the University of Warwick; Zheng Liu and Kazu Suenaga from National Institute for Advanced Industrial Science and Technology (AIST), Research Centre for Advanced Carbon Materials, Tsukuba, Ibaraki Japan; and Laura M. Perkins and Ian J. Shannon from the University of Birmingham.
Provided by University of Warwick




















