Different roads to diabetes
(PhysOrg.com) -- A specific genetic variant puts individuals of Asian ancestry at risk of developing diabetes -- but not their European counterparts.
Type 2 diabetes is relatively widespread in Japan, and that nation’s Ministry of Health, Labour and Welfare estimates that nearly one-third of all individuals over the age of 40 are either diabetic or pre-diabetic. Intriguingly, Japanese patients as a whole are less prone to obesity, a condition commonly associated with onset of type 2 diabetes in the Western world, indicating that there may be some significant differences in disease pathology between these two groups.
Two primary mechanisms contribute to the onset of type 2 diabetes. Fat, muscle and liver cells lose the ability to respond to the hormone insulin, a state known as ‘insulin resistance’ that greatly reduces the efficiency with which excess glucose is taken up from the bloodstream; in parallel, the capacity of the pancreatic beta cells to produce and secrete additional insulin is impaired (Fig. 1). However, Shiro Maeda of the RIKEN Center for Genomic Medicine in Yokohama points out that the relative importance of these mechanisms appears to differ between Eastern and Western populations. “Accumulating clinical evidence suggests that disability of insulin secretion contributes more to the pathogenesis of Japanese type 2 diabetes,” he says, “whereas insulin resistance seems more important for European type 2 diabetes.”
Getting the big picture
Very little is known about the genetic-level differences in pathology between the Japanese and Europeans. From several previous genome-wide association studies (GWAS), geneticists have identified several small sequence changes, also known as single-nucleotide polymorphisms (SNPs), which might be located near or within genes involved in type 2 diabetes. They have also identified several of these ‘susceptibility loci’. However, with the exception of a few small-scale Japanese studies, nearly all of these data were obtained exclusively from individuals of European ancestry.
In an effort to collect more information about Japanese-specific risk factors, Maeda and Takashi Kadowaki of The University of Tokyo recently headed up a large GWAS that matches the scale of its European counterparts both in terms of the numbers of subjects involved and in the number of genomic markers examined1. According to Maeda, their study of some 5,000 type 2 diabetes subjects and 3,000 controls for 459,359 SNPs, is one of the largest sample sizes for a single GWAS worldwide.
Maeda, Kadowaki and colleagues subjected 98 candidate SNPs with the strongest association to type 2 diabetes to an additional round of analysis in a second, independent, set of disease and control cohorts. Based on these data, they identified statistically significant disease linkage for SNPs at a number of different genomic loci (Fig. 2). One of these SNPs, KCNQ1, was identified in both of the previous Japanese GWAS as a risk factor in both Asians and Europeans, and this gene appears to participate in the regulation of glucose-induced insulin secretion.
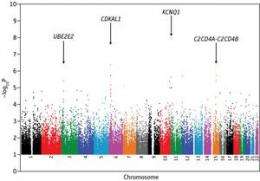
The researchers’ study also flagged SNPs at two additional loci for which no association to type 2 diabetes had been previously reported. Both of these loci were subsequently validated in yet a third round of genomic screening, reinforcing the likelihood of their connection to diabetes.
Enigmatic risk factors
One of the newly identified loci, UBE2E2, encodes an enzyme that targets proteins for destruction by marking them with individual molecules of the small protein ubiquitin. It is expressed in a variety of tissues, including the liver, pancreas, muscle and fat. Based on several recent studies, researchers have suggested that the ubiquitination pathway may contribute to the efficient synthesis and secretion of insulin. Maeda, Kadowaki and colleagues noted that study subjects with the diabetes risk-associated UBE2E2 allele appeared to exhibit impairments in insulin regulation.
Strikingly, although the SNPs at this locus were also determined to be associated with type 2 diabetes for three other East Asian populations, in addition to various Japanese study groups, this association was not statistically significant for two European groups, consisting of a total of 6,980 subjects and 8,615 controls. “Although this population-specific effect needs to be validated further, the present study is the first to show the existence of a disease-susceptibility locus [for diabetes] in a population-specific manner with genome-wide significant levels of association,” says Maeda.
Despite escaping detection in previous large-scale GWAS, the second newly identified locus, C2CD4A-C2CD4B, showed significant association with type 2 diabetes in both Asian and European cohorts. C2CD4A-C2CD4B produces a pair of factors that appear to contribute to the maintenance of cell structure, and although relatively little is known about their function, both factors are expressed in many of the same tissues as UBE2E2. This represents the first indication that they might contribute to the pathology of type 2 diabetes.
Digging deeper
Kadowaki, Maeda and colleagues are now scanning these two loci more carefully in an effort to identify potential mechanisms by which sequence variants in these regions might contribute to onset of diabetes. Maeda indicates that they are particularly keen to understand the biological basis for the apparent ethnicity-specific disease association of SNPs in the UBE2E2 locus.
At the same time, the identification of a novel locus that appears to contribute to risk of diabetes across population lines suggests that there may be a number of other susceptibility genes waiting to be discovered. “The identification of C2CD4A-C2CD4B as a common locus is really surprising, because this important locus has been missed in European GWAS,” says Maeda, “[and] we are now participating in international collaborations, both East Asian and trans-ethnic, to identify additional type 2 diabetes susceptibility loci.”
More information: Yamauchi, T., Hara, K., Maeda, S., Yasuda, K., Takahashi, A., Horikoshi, M., Nakamura, M., Fujita, H., Grarup, N., Cauchi, S. et al. A genome-wide association study in the Japanese population identifies susceptibility loci for type 2 diabetes at UBE2E2 and C2CD4A-C2CD4B. Nature Genetics 42, 864–868 (2010).
















