Researchers identify potential target for breast cancer therapy
(PhysOrg.com) -- Overexpression or hyperactivation of ErbB cell-surface receptors drives the growth of many breast cancers. Drugs, like Herceptin, that block the receptors’ signals halt tumor progression in some patients. However, not all patients’ tumors respond, with some becoming resistant over time. Different drugs that interfere with other steps in the signaling pathway may improve the response of patients, yet little is known about these molecules.
Now, Marcelo G. Kazanietz, PhD, professor of Pharmacology at the University of Pennsylvania School of Medicine and colleagues, report that a protein called P-Rex1 is crucial for signal transmission from ErbB receptors. What’s more, they found that P-Rex1 is overexpressed in nearly 60 percent of breast cancer samples tested and patients whose tumors express P-Rex1 were more likely to develop metastasis, compared with those whose tumors did not express P-Rex1.
“We identified a downstream target of the ErbB receptors which seems to be crucial for cancer cell proliferation, migration, and metastasis,” Kazanietz says. “Understanding how this pathway works should allow us to find new drugs or therapeutic approaches in the future.”
The team’s research is featured on the cover of the December 22 issue of Molecular Cell.
A Well-Known Family
The ErbB family of receptors is well known in the cancer world. The family includes the epidermal growth factor receptor (EGFR, also known as ErbB1); ErbB2 (also known as HER2/neu), which is the target of Herceptin; as well as ErbB and ErbB4.
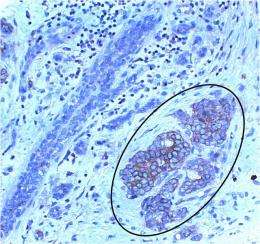
Previous work from Kazanietz and others suggested the receptors might rely on small proteins in the Rac pathway to help transmit their signal. To find out if that was the case, Kazanietz and colleagues examined human breast cancer cell lines and found that one Rac pathway protein, P-Rex1, is overexpressed in numerous cell lines compared with normal mammary cells. The team also found that P-Rex1 is present in some breast tumors, particularly those that express the Her2/neu receptor or estrogen receptor and belong to the luminal subtype.
“We found that about two-thirds of the patient samples had very high levels of P-Rex1 expression in tumor cells in their lymph nodes,” Kazanietz says. “There seems to be a correlation between P-Rex1 expression in the tumor cells and the capacity of these cells to metastasize. And since P-Rex1 is likely to be essential for cell migration and migration is essential for metastasis, we believe blocking this pathway could reduce the risk of metastasis.”
P-Rex1 may be important for several other cancer-promoting pathways. For example, estrogen-receptor signaling also appears to rely on P-Rex1, which means that targeted inhibitors of P-Rex1 might improve responses to anti-estrogen therapies such as tamoxifen.
The authors also found that P-Rex1 is also used by another receptor, CXCR4, which has recently shown up in many cancer studies. Despite being used in numerous pathways during cancerous growth, P-Rex1 is not expressed in many normal tissues. “That gives us a very good target. It is really cancer specific,” Kazanietz says. “What’s more, as P-Rex1 is expressed in some subtypes of breast tumors, it may be an excellent prototype for future personalized medicine.”
Preclinical data from other groups supports the importance of the Rac pathway in cancer, according to Kazanietz. And small molecule inhibitors that block proteins in the Rac pathway appear to have strong anti-cancer effects in model systems, though their use in patients still needs to be tested.













