Shedding light on the elegant mechanisms that control the push and shove of cells in living organisms
The many amazing photomicrographs on display at the entrance to the Electron Microscope Laboratory in the RIKEN Center for Developmental Biology (CDB) were all taken by laboratory leader Shigenobu Yonemura and his colleagues. In addition to its own research program, the laboratory also offers technical assistance with electron microscopic analysis to researchers at other laboratories in the CDB. “Moving cells seem to have a life of their own. I have long been fascinated by the motion force generated by cells,” says Yonemura. His team’s recent clarification of the mechanism by which adjacent cells pull and pull against each other has attracted considerable attention.
Yonemura clearly remembers his days as a graduate student examining the development of the sea urchin. “The fertilized egg of a sea urchin cleaves and replicates, doubling the number of cells in sequence to build up a spherical body. At one point, part of the sphere suddenly sinks, taking surface cells into the body itself. This process is known as archenteron invagination. I was fascinated by the change, in which it looks as if the surface cells move under their own volition. Since then, I have been interested in the force generated by cells.”
That force is produced by two proteins: actin and myosin. Individual actins occur in spherical form, with many actins joining together to form actin filaments. As myosins move on the filaments, a force that pulls the filaments is produced, which in turn allows the cells to move or change their form. “It is not necessary for the cells to be in the process of morphogenesis, such as during archenteron invagination, for them to generate a force. For example, adjacent epithelial cells are constantly pulling at each other.”
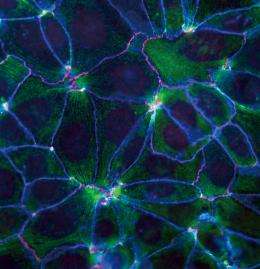
Epithelial cells play an important role in separating the internals of a living body from the outer world by arranging themselves in a sheet to form the surface of the skin and the gastrointestinal tract. Adjacent epithelial cells are linked together by adhesion apparatus (Figs 1, 2). The key component of this apparatus is cadherin, a protein discovered in 1982 by Masatoshi Takeichi, director of the CDB. Cadherin penetrates the cell membrane, with one end exposed to the cell surface, and the other end protruding into the cell. Bound to the end of the cell are α-catenin and β-catenin. As the actin filaments bind to the α-catenin and the myosin acts on the filaments, a pulling force is produced.
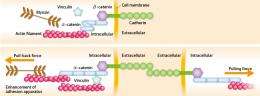
“As cadherin adheres to the same protein in an adjoining cell, the adjoining cell is pulled by tensile force. If that pulling force continues, however, the cell is deformed. A good balance cannot be achieved unless the adjoining cell pulls back. When pulled strongly, the cell pulls back strongly, and when pulled weakly, it pulls back weakly. Believing in the existence of a mechanism behind this, I have been trying to find what it is, and recently succeeded in clarifying it. The key player proved to be α-catenin."
The mechanism is remarkable. Each cell contains α-catenin, which assumes a bent structure (Fig. 2). When pulled by an adjoining cell, the bent portion stretches. The vinculin molecule then binds to the α-catenin, and as the actin filaments continue to bind to the vinculin, the adhesion apparatus is enhanced and a pulling-back force is produced.
“This can be compared to a tug-of-war in which one team is about to lose the game as it is pulled with a strong force by its opponent, then many helpers come to join and pull back strongly,” says Yonemura. When the opponent's pulling force weakens, the helpers are no longer necessary. The α-catenin then bends again, and the vinculin and actin filaments leave the adhesion apparatus, restoring the original state. “From this system, I realized that α-catenin is responsible for two tasks: sensing the forces and enhancing the adhesion apparatus.”
When a cell is pulled by an adjacent cell, it must immediately pull back at the same location. However, transducing information about such a response by the release of a signal transmitter, as occurs in many biological processes, would not provide the responsiveness required to balance rapid changes in pulling location and strength. “It is reasonable that one molecule plays the dual roles of sensing the forces and enhancing the adhesion apparatus. We have arrived at a definite answer to the inveterate problem of how cells perceive forces and respond to them.”
Yonemura is planning to make observations of how the α-catenin structure changes to allow binding to the vinculin, and to quantify the pulling force required to cause morphological changes in the cells.
“Pulling at each other is very important for cells. I think that mutual pulling is utilized in, for example, sensing whether an adjoining cell is alive or dead,” says Yonemura.
Dead epithelial cells are quickly eliminated from the sheet structure. “The mechanism by which cells sense the death of adjoining cells remains unclear. Although it is said that dead cell detection may be achieved by molecular exchanges on the cell surfaces, I do not think that’s the whole story.”
To investigate the mechanism behind the sensing of dead cells, Yonemura artificially damaged epithelial cells and examined them in the process of repair. Similar experiments undertaken before had not yielded good results because the experimentally inflicted damage affected a larger range than intended due to cell scratching. Yonemura succeeded in selectively killing a target cell by laser irradiation. His technique has made it possible to make extensive observations of the behavior of cells adjacent to the dead cell (Fig. 3).
“When a cell dies, a change begins to occur in the cells adjacent to the dead cell. Actin filaments and myosins gather on the surfaces in contact with the dead cell as if they were joining hands to form a ring surrounding the dead cell. The ring gradually shrinks, and eventually the dead cell is ejected from the sheet.”
This accumulation of actin filaments and myosins begins as soon as a cell dies. “The repair of epithelial cells cannot begin immediately as it does unless actin filaments and myosins are involved in sensing cellular death,” says Yonemura. “Cells are constantly pulling at each other. When an adjacent cell has died, however, the living cell in question continues to pull the dead cell, but is no longer pulled back. It is through this process that living cells may sense the death of an adjacent cell. Such a process would ensure that death signals are detected earlier and more accurately, allowing the elimination of the dead cell to start more quickly than if cellular death were detected by molecular exchange on cell surfaces.”
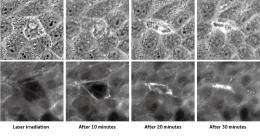
However, that may not be the full story. “When actin filaments and myosins have formed a ring surrounding the dead cell, the forces remain balanced. It is natural that the process ceases in that state. In actuality, however, the ring continues to shrink further to eliminate the dead cell. There must be another, unknown mechanism that breaks the balance of forces. Clarification of the mechanism behind the series of processes from the sensing of cellular death to its elimination is an issue that remains to be addressed.”
Of course, gene expression and molecular signal transduction are vitally important to the functioning of cells. “Although this is true, there is also a wide variety of mechanisms that act like machinery in cells. I am much more interested in those types of machinery. I want to discover these exquisite mechanisms, which are remarkably simple and controlled by feedback,” says Yonemura.
In addition to its own research program, the Electron Microscope Laboratory provides technical support for electron microscopic analysis to researchers at the CDB. Yonemura also provided such support while conducting his own research at the Laboratory for Cellular Morphogenesis in the CDB, for which he served as team leader between 2001 and 2006. Even after the team was renamed the Electron Microscope Laboratory in April 2007, its tasks have continued basically unchanged.
“Only a few researchers are good at using electron microscopes,” says Yonemura. Electron microscopes have a resolution of as fine as 0.1 nanometers, compared with the much coarser resolution of 200 nanometers achievable using optical microscopes, making it possible to observe much smaller features. “It is likely that users realize the performance of the electron microscope but tend to view it as something that is difficult to operate, possibly because of the large amount of instrumentation, so they are reluctant to use it. Myself and two technical staff members provide technical assistance on their use.”
At the Electron Microscope Laboratory, members manage two transmission electron microscopes and one scanning electron microscope, as well as peripheral equipment including two microtomes for preparing sample sections. “We want researchers to be able to examine as many samples as possible, so we chose to install more pieces of standard equipment, rather than one or two highly specialized, high-performance units.”
Currently, the laboratory provides assistance to about 20 research projects annually. In some cases, support is provided for all steps from the preparation of resin-fixed sectional samples to examination and photographic recording. In other cases, it is only necessary to provide instructions on how to use the instruments. Many different types of materials are brought into the laboratory, including mice, drosophila fruit flies, nematodes, zebrafish and chickens. The types of tissue examined are also diverse, and include embryos, nerves and kidneys. “New types of samples can be quite difficult to handle the first time, so it can take a lot of time to prepare them properly. However, since all trial-and-error experimentation represents the accumulation of knowhow, we always endeavor to work steadily without rushing. State-of-the-art techniques are not the only key to success in this field. We must have a broad range of knowledge about the wide variety of tissues of various organisms, and be able to hold scientific discussions with the users.”
When providing support, Yonemura discusses the project and desired goals with the requestor. “We do this to determine whether the target is in fact suitable for analysis using an electron microscope. Electron microscopy only allows a relatively narrow range of examination compared with optical microscopy. The sample must also be fixed in resin, and this is a time-consuming procedure. Our discussions sometime lead us to recommend use powerful optical microscopes. To ensure the best outcomes, we make it a rule to first discuss things in detail with the requestor.”
Yonemura’s support role also has its own benefits. “The support work allows me to get involved in many kinds of research that I would otherwise never do myself, and it provides me with opportunities to see the very latest achievements before they are published. For me, the greatest fascination is in meeting many different people. It makes me very happy to be able to continue to do my own research and at the same time balance that with our support work.”
Provided by RIKEN