Simple lithium good for many surprises

(PhysOrg.com) -- At first glance, lithium should be a simple atomic system. It is the lightest solid element and with just three electrons, it should exhibit simple, crystalline structures. However, an international team of scientists has shown that under high pressure, lithium “prefers” the liquid state, and that it turns out to be the elemental metal with by far the lowest melting point. At high pressure, lithium also undergoes a series of phase changes into surprisingly complex structures. The results of these experiments performed at the U.S. Department of Energy Office of Science’s Advanced Photon Source (APS) at Argonne, and at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France, were published Nature Physics.
At ambient conditions, lithium forms rhombohedral crystals, which at higher temperatures and pressures transform to face-centered cubic and then body-centered cubic crystals – all three among the simplest known crystal structures. But what happens to lithium at very high pressures? In the past few years, intriguing deviations from simple metallic behavior were observed, for example a metal to semiconductor-transition and even superconductivity at 17K.
Nevertheless, the overall picture of the lithium phase diagram remained patchy, motivating a systematic study by researchers from The University of Edinburgh, the ESRF, and the Carnegie Institution of Washington who have mapped the lithium phase diagram at high pressures up to 1.3 Mbar, and over a wide temperature range between 77K and 300K.
Whereas the melting point of a material usually rises under pressure, and even the lightest gaseous elements, hydrogen and helium, melt at 1000K and 0.5 Mbar, lithium remains liquid at this pressure down to temperatures as low as 190K. This is by far the lowest melting temperature observed for any material at this pressure.
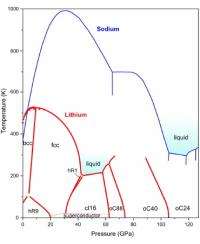
There are more surprises: Above 0.6 Mbar, lithium adopts three novel, complex crystal structures not previously observed in any element with 40, 88, and 24 atoms per unit cell. The most complex ones, with 40 and 88 atoms, were even never predicted theoretically. The scientists suggest that the overall appearance of the lithium phase diagram and particularly the anomalously low melting temperatures are due to quantum effects starting to play the dominant role at high compressions. They also speculate that a ground metallic liquid state, which has been predicted but never observed for hydrogen, and which should exhibit highly unusual properties, might be constructed on the basis of the lithium-rich compounds.
The experiments were carried out using powder diffraction and single-crystal diffraction at the ESRF High-Pressure Beamline ID09A, and at the High Pressure Collaborative Access Team (HP-CAT) 16-ID-B beamline at the APS, where, in addition, critical cryogenic techniques were refined.
The research team had to address several experimental challenges: handling a diamond anvil cell in a cryostat; ensuring a wide opening angle to allow for single-crystal diffraction; and last but not least, coping with the high reactivity of lithiumm in particular when in the liquid phase.
More information: Christophe L., et al. “Cold melting and solid structures of dense lithium,” Nat. Phys., published online: 9 January 2011. DOI: 10.1038/NPHYS1864
Provided by Argonne National Laboratory



















