The generality of surface vanadium oxide phases in mixed oxide catalysts
In the spirit of the physicist's pursuit of a 'theory of everything,' Israel E. Wachs, the G. Whitney Snyder Professor of Chemical Engineering at Lehigh University, has published a paper entitled "The generality of surface vanadium oxide phases in mixed oxide catalysts."
It is common knowledge throughout the heterogeneous catalysis community that reactions take place at the surface of metal oxide catalysts, rather than in the bulk (inside) of the catalyst. Thus, the goal of fundamental catalysis research focuses on developing structure-activity relationships based on the surface metal oxide phases present, ultimately allowing for the rational design of improved heterogeneous catalysts from the ground up.
This paper discusses research on vanadium oxide-containing catalysts performed in the past 30 years using a variety of techniques: IR and Raman vibrational spectroscopies, CH3OH-temperature programmed surface reaction (TPSR) spectroscopy and steady-state oxidation reactions. The paper shows that surface VOx phases, two-dimensional vanadium oxide overlayers, are a general phenomenon in vanadium-containing mixed oxide catalytic materials and that they also control the catalytic properties.
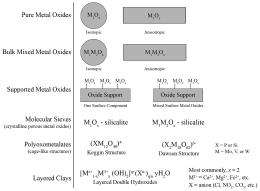
Mixed oxide catalysts consist of many different metal oxide arrangements, as depicted in the figure. Bulk oxides consist of either pure oxides (e.g., V2O5) or mixed oxides that can exist as either stoichiometric compounds (e.g., FeVO4) or as solid solutions (e.g., VxTi1-xO2). Supported metal oxides involve the impregnation of metal oxides onto high surface area supports (e.g., pure oxides, mixed oxides, zeolites, or molecular sieves). Polyoxometalate (POM) clusters are nanometer sized mixed oxide clusters consisting of a central XO4 unit (PO4, SiO4, etc.) that are surrounded by 12 or 18 O=MO5 units (M = V, Mo, W, Cr, etc.).
Supported Metal Oxides
On pure oxide supports, isolated O=VO3 species are almost exclusively present at low surface coverages (<2 V atoms/nm2). At higher surface coverages, polymeric surface (O=VO3)n species become the predominant vanadium oxide phase. It was found that for reactions involving only one participating O atom, the catalytic reaction rate is essentially independent of the surface O=VO3 coverage. For reactions involving the participation of more than one O atom, the catalytic reaction rate increases with surface O=VO3 coverage. The same trend in surface O=VO3 species structure is also observed for mixed oxide supports. A detailed discussion of the mixed oxides that possess surface O=VO3 species is provided in the article.
Characterization of the molecular sieve V-silicalite, amorphous mesoporous supported V2O5/SiO2, and vanadium oxide impregnated ZSM-5 zeolites by Raman spectroscopy and solid state 51V NMR revealed the same isolated O=VO3 species is present as seen on pure and mixed oxide supports. At very high vanadium coverage, crystalline V2O5 nanoparticles form. The situation for layered clays and hydrotalcite materials is slightly more complex due to the presence of intercalated vanadium oxide in the interlayer of these layered hydroxides. The interfacial vanadia species, however, were found to be no different than those present in supported vanadium oxide catalysts. In the case of V-containing POMs, the substituted VOx units take on a distorted O=VO5 coordination, where four oxygen atoms bond to adjacent cations and the fifth oxygen atom weakly bonds to the central heteroatom.
The remainder of the article, published in Applied Catalysis A: General, volume 391, discusses similar surface VOx species and catalytic activity trends on unsupported bulk mixed oxides. “This paper demonstrates for the first time that surface metal oxide phases are pervasive in mixed oxide materials and also represent the catalytic active sites responsible for chemical transformations by heterogeneous mixed oxide catalytic materials,” states Israel Wachs.
More information: The article is published in Applied Catalysis A: General, 2011, 391, 1-2, 36-42. Article URL: dx.doi.org/10.1016/j.apcata.2010.08.048
Provided by Lehigh University

















