February 25, 2011 feature
Scientists create one-dimensional ferroelectric ice
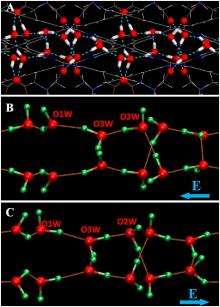
(PhysOrg.com) -- Everyone knows that when water freezes, it forms ice. But a lesser known fact is that there is not one, but many different kinds of ice, depending on the way the ice crystals are arranged. In a new study, a team of chemists has developed a new method for synthesizing a type of ferroelectric ice, which is crystallized so that all of its bonds line up in the same direction, producing a large electric field.
The researchers, including Hai-Xia Zhao, Xiang-Jian Kong and La-Sheng Long, along with their coauthors from Xiamen University in Xiamen, China, and Hui Li and Xiao Cheng Zeng from the University of Nebraska in the US, have published their study in a recent issue of the Proceedings of the National Academy of Sciences.
Every water molecule carries a tiny electric field. But because water molecules usually freeze in a somewhat random arrangement, with their bonds pointing in different directions, the ice’s total electric field tends to cancel out. In contrast, the bonds in ferroelectric ice all point in the same direction at low enough temperatures, so that it has a net polarization in one direction that produces an electric field.
Ferroelectric ice is thought to be extremely rare; in fact, scientists are still investigating whether or not pure three-dimensional ferroelectric ice exists in nature. Some researchers have proposed that ferroelectric ice may exist on Uranus, Neptune, or Pluto. Creating pure 3D ferroelectric ice in the laboratory seems next to impossible, since it would take an estimated 100,000 years to form without the assistance of catalysts. So far, all ferroelectric ices produced in the laboratory are less than three dimensions and in mixed phases (heterogeneous).
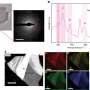
In the new study, the scientists have synthesized a one-dimensional, single-phase (homogeneous) ferroelectric ice by freezing a one-dimensional water ‘wire.’ As far as the scientists know, this is the first single-phase ferroelectric ice synthesized in the laboratory.
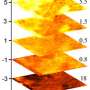
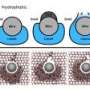
To create the water wire, the researchers designed very thin nanochannels that can hold just 96 H2O molecules per crystalline unit cell. By lowering the temperature from a starting point of 350 K (77°C, 171°F), they found that the water wire undergoes a phase transition below 277 K (4°C, 39°F), transforming from 1D liquid to 1D ice. The ice also exhibits a large dielectric anomaly at this temperature and at 175 K (-98°C, -144°F).
“We know the freezing point should be different from normal water because the water is confined to nanochannels and not in a normal environment,” Zeng told PhysOrg.com. “Why 1D water has a higher temperature in this case is still an open question.”
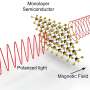
As the scientists explained, the hydrogen-bonding interactions among H20 molecules in the water wire and the nanochannel play an important role in the ferroelectricity of the ice. While the hydrogen bonds between the water and nanochannel do not break, the remaining hydrogen atoms in the ice rotate under an opposite electric field. As a result, the polarity of the ferroelectric ice can be reversed by reversing the external electric field, a property not seen in everyday water and ice.
Overall, the production of a 1D, single-phase ferroelectric ice using water confined to a nanochannel provides a new way to synthesize ferroelectric materials. The new method could also help scientists better understand the unique properties of ferroelectric ice, which could have applications in the biological sciences, geoscience, and nanoscience. As Zeng noted, ferroelectric ice could potentially have electrical applications, with the efforts of engineers working in nanotechnology.
“[The study] shows that the freezing of water can be greatly affected by the confinement and water/surface interaction,” Zeng said. “So knowledge and insights gained through research in this field will help scientists to control some properties of water through designing different confinements.”
More information: Hai-Xia Zhao, et al. “Transition from one-dimensional water to ferroelectric ice within a supramolecular architecture.” PNAS Early Edition. DOI:10.1073/pnas.1010310108
Copyright 2010 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.