Tough crystal nut cracked: Correct prediction of all three known crystal structures of a sulfonimide
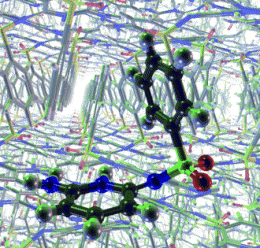
(PhysOrg.com) -- It's not just the type of molecules a material is made of, the way in which they are arranged in space is important too. For many organic molecules, multiple crystal structures are known, and their physical properties can differ significantly. For example, a drug can be effective in one crystalline form but much less effective in another because it doesn't dissolve fast enough. Unfortunately, it has not been possible until recently to reliably predict crystal structures by using computer simulations. Frank Leusen and his co-workers at the University of Bradford (UK) are making significant progress on this front. As the scientists report in the journal Angewandte Chemie, they successfully used a quantum mechanical approach to predict the three known crystal structures of a sulfonamide.
Small differences in the production conditions, such as variations in pressure or temperature, can be enough to cause fine chemicals, such as pharmaceuticals, pigments, explosives, or agrochemicals, to crystallize in a different form. This can lead to problems with the production process or to undesirable product properties. It is correspondingly important to know which crystal structures are possible.
Scientists use computational chemistry methods to obtain information about molecular structures and crystallization processes. However, taking all of the parameters into account would exceed current computational capacities. “Precise, reliable predictions of the crystal structures of organic molecules have remained somewhat of a Holy Grail for crystallography,” says Leusen.
An international project regularly organizes blind studies in which research groups are asked to predict crystal structures. In 2007, Leusen and two co-workers were able to successfully predict the crystal structures of all four test compounds by using a quantum mechanical approach. A team led by Leusen then took on another test compound, a sulfonamide, which was the subject of a blind study in 2001; none of the participating teams was able to predict the crystal structure at the time. Interestingly, two additional, previously unknown crystal structures of this sulfonamide were discovered after the study. “By using the computational process developed by Marcus Neumann at Avant-garde Materials Simulation in Freiburg, Germany, we were able to correctly predict all three crystal structures,” says Leusen.
“Even though it is currently not possible to predict the outcome of a specific crystallization experiment under specific boundary conditions,” explains Leusen, “our results demonstrate that precise calculations of the lattice energy are sufficient to model crystallization thermodynamics and thus predict the different crystal structures of small organic molecules.”
More information: Frank J. J. Leusen, Molecule VI, a Benchmark Crystal-Structure-Prediction Sulfonimide: Are Its Polymorphs Predictable? Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201007488
Provided by Wiley