Timid and shy or bold and welcoming, water behaves in unexpected ways on surfaces

It's ubiquitous. It's universal. And it's understood—not! Water's choices in a given situation often defy scientific predictions. When expected to bond with other water molecules, it shuns them. When expected to ignore a surface, it becomes deeply attached. However, research at Pacific Northwest National Laboratory has revealed why one of the simplest and most important molecules on the planet makes some of the decisions it does.
Controlling the interactions between water and surfaces could alter a great deal of how we design technologies and solve the nation's energy problems. Water's interactions with surfaces are important to the next generation of fuel cells, converting biomass to fuels, and understanding cloud formation for weather patterns and global climate change.
Before these applications can be realized, water must be understood. The theories of how water works must agree with the experimental data, and vice versa. Two studies by scientists at PNNL have overturned decades of conventional wisdom about water. Drawing upon expertise in different scientific disciplines and new instruments, the researchers built computational models that captured their understanding for others to use.
You build models of the world every day. You use them to predict the choices someone will make. For example, you want to ask that cute barista on a date. You consider a lot of variables that would influence the outcome. For example, are they more likely to say "yes" if you ask in person or send a text message? Would sending yellow roses be romantic or over the top? Should you suggest dinner at the fancy new bistro or a simple picnic in the park? You codify your observations and experiences to predict outcomes. That's modeling.
Scientists do the same with water and surfaces. But, because liquid water and steam are still too difficult to predict with today's instruments, scientists work with frozen water. It is simply easier to study. The ice has the same structure as water in other forms. And, when working at the molecular level, ice isn't stationary. It moves and flows, even at very low temperatures.
"If you want to have a theory and an understanding of how water interacts with surfaces, you must have a model case," said Dr. Greg Kimmel, an American Physical Society Fellow at PNNL who has led key water studies. "The model is the test bed for sharpening our understanding of the underlying physics of what happens. With it, we can test—in detail—the structures. But, if you can't even do ice, how are you going to have confidence in the structures you define for fuel cells?"
As an example, in the 1980s and 1990s, scientists believed that one of the best ways to grow a perfectly smooth, very thin layer of ice was to start with platinum. Scientists created clean, well-characterized surfaces and added water. However, the experimental results didn't quite match the predictions; they didn't contradict them either.
Flash forward to 2005 and a team of scientists at PNNL—they wanted to understand conflicting studies about water. They were especially interested in an intriguing study from Stanford University. So, the PNNL team created a smooth platinum wafer and added water. They examined the surface using a technique called rare gas physisorption. This technique propels atoms of the rare gas krypton at the ice layers and records how they respond. The results, however, did not match their predictions.
So, they went back to the basics and re-examined their assumptions about water's behavior. They determined that the first thin layer, or monolayer, of water spread across the platinum surface as they had expected. But, the subsequent water molecules clumped together, afraid to expand across the surface and none too thrilled about the water layer below them.
"In other words, the first layer of water is hydrophobic," said Kimmel.
The V-shaped water molecules preferred to bind to the platinum. By binding to the platinum, the first water layer became very unattractive to the incoming water molecules. So, the next layer of water beaded up, forming icy patches. As more layers of water were added, the islands slowly grew and eventually covered the surface.
"When you look at it, the standard model that had been around for 20 to 30 years is just not a good model for how water grows," said Kimmel.
The discovery of self-loathing water led the team to ask what would happen if layers of water were grown on top of a truly hydrophobic surface, one that has absolutely no interest in water.
So, the scientists looked to graphene, a thin slice of carbon.
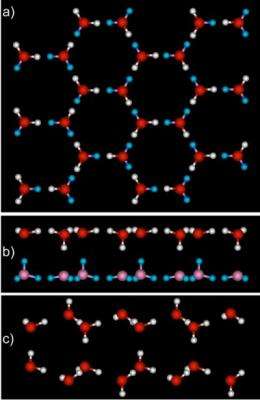
In 2009, a team at PNNL, including scientists from the 2005 study, placed graphene on top of the platinum. The surface does not interact strongly with the water, merely providing a playground for the water molecules to meet in two dimensions. Then, they introduced a small amount of water vapor onto the surface at very low temperatures, around 125 K or the approximate temperature of an evening on the moon.
Next, the team used rare gas physisorption in combination with low-energy diffraction, a technique that sends waves of slow-moving electrons at the surface. How those electrons bounce off the surface tells the researchers about the structure of the material. They expected the water to form icy patches that did not cover the surface.
Surprisingly, they found a smooth, two-layer-thick film of ice had grown on the graphene. The water stretched out evenly across the surface and bonded with its neighboring compatriots. In contrast to platinum, the ice was flat and two layers thick. The angles between the atoms in the water molecules were stretched or compressed compared to normal ice. "This makes for stressed ice," said Kimmel.
The team subjected the ice to infrared spectroscopy and determined that the water molecules were in unusual configurations. With these results, PNNL theoreticians Dr. Christopher Mundy and Dr. Marcel Baer used EMSL's supercomputer to determine that in each layer of the ice, the water molecules formed slightly larger rings than normal. These six-sided rings stacked on top of each other. They also calculated that each water molecule formed four hydrogen bonds-three with other molecules in the same layer, and one with water in the layer on top.
These studies have changed the fundamental concept of water, explaining disparate results and confounding theories. In addition, this work has become part of a larger movement. Scientists at major institutions are making great advances in understanding water to control it for industrial processes, chemical reactions, and biochemical systems.
But, by no means is this the conclusion to water's story. At PNNL, scientists are exploring the reactions of water on a common catalyst, titanium dioxide. While the structure of water and the surface are well known and fairly straightforward, they aren't expecting the interactions to be simple.
"In a lot of cases, water gets pretty complicated pretty fast," said Kimmel. "That's what keeps this research so interesting."
More information: References:
Kimmel GA, et al. 2009. "No Confinement Needed: Observation of a Metastable Hydrophobic Wetting Two-Layer Ice on Graphene." Journal of the American Chemical Society131(35):12838.
Kimmel GA, et al. 2006. "Layer-by-layer Growth of Thin Amorphous Solid Water Films on Pt(111) and Pd(111)." Journal of Chemical Physics 125, 044713.
Kimmel GA, et al. 2007. "Crystalline Ice Growth on Pt(111) and Pd(111): Non-Wetting Growth on a Hydrophobic Water Monolayer." Journal of Chemical Physics 126, 114702.
Kimmel GA, NG Petrik, Z Dohnálek, and BD Kay. 2005. "Crystalline Ice Growth on Pt(111): Observation of a Hydrophobic Water Monolayer." Physical Review Letters 95(16):166102.
Provided by Pacific Northwest National Laboratory



















