Engineer to launch bacteria into space aboard the final mission of space shuttle Atlantis

There will be some very interesting passengers on the final mission of the NASA Space Shuttle Atlantis scheduled to launch July 8, 2011: thousands of bacteria.
Cynthia Collins, assistant professor of chemical and biological engineering at Rensselaer, is leading a series of experiments called Micro-2A that will be aboard the shuttle during its scheduled 12-day mission. The research seeks to understand how microgravity changes the way potentially dangerous bacteria grows. In particular, the research will examine how they form difficult-to-kill colonies called biofilms. The research has important implications for protecting astronauts while they are in space in enclosed and difficult-to-clean spaces, such as the International Space Station, or during extended space missions deeper into our solar system. It also provides new information in the fight against ever-more virulent bacterial infections such as staph, food poisoning, sepsis, and pneumonia.
Partnering with Collins on the project are nanobiotechnology expert Jonathan Dordick, the Howard P. Isermann Professor of Chemical and Biological Engineering at Rensselaer and director of the Rensselaer Center for Biotechnology and Interdisciplinary Studies, and thin films expert Joel Plawsky, professor in the Department of Chemical and Biological Engineering. The NASA Ames Research Center is funding the experiment.
This is the second time that Collins’ research will be included on the shuttle. Her research on bacteria was also aboard the shuttle mission that launched May 14, 2010. Collins has been analyzing the results of this previous work and will use this new series of experiments to test some of the results she has seen.
“We are clearly seeing altered biofilm formation during space flight,” she said. “There are some clear differences between the amount of biofilm formed in normal gravity and microgravity. These differences also appear to be organism dependent, with different organisms responding very differently to the environment in space.”
The bacteria that Collins will include are Pseudomonas aeruginosa and Staphylococcus aureus. These bacteria are responsible for more hospital-acquired infections than any other, according to Collins. The Center for Disease Control places hospital-acquired infections such as those caused by these bacteria as the fourth leading cause of death in the United States.
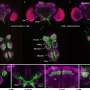
Biofilms are complex, three-dimensional microbial communities. Most biofilms, including those found in the human body, are harmless. Some biofilms, however, have been shown to be associated with disease. Researchers like Collins are discovering that the bacteria within these colonies have very different properties, including increased resistance to antimicrobials, compared with bacteria not encased in a biofilm.
Collins and her team will send up 16 devices, called Group Activation Packs (GAPs) and each containing eight vials of bacteria, aboard the shuttle. The GAPs and other hardware used by the Collins and her team were developed by BioServe Space Technologies. While in orbit, astronauts will begin the experiment by manipulating the sealed GAPs and combining the bacteria with nutrients and a surface on which they can form biofilms. At the same time, Collins will perform the same actions with identical GAPs on Earth at the Kennedy Space Center in Florida. After the shuttle returns, her team will compare the resulting biofilms to see how the behavior of bacteria and development of biofilms in microgravity differs from the Earth-bound control group.
In addition, the research team will also test if a newly developed, antimicrobial surface — developed by Dordick at Rensselaer — can help slow the growth of methicillin resistant Staphylococcus aureus, or MRSA, on Earth and in microgravity. Actual MRSA, the bacteria responsible for antibiotic-resistant infections, will not be used for the safety of those on board. A different and safer strain of bacteria with similar properties will serve as a proxy. The new surface developed by Dordick utilizes an enzyme found in nature and kills 100 percent of MRSA within 20 minutes of contact.
The new technology marries carbon nanotubes with lysostaphin, a naturally occurring enzyme used by non-pathogenic strains of staph bacteria to defend against staph growth. The resulting nanotube-enzyme biomaterial can be mixed with any number of surface finishes. In tests, it was mixed with ordinary latex house paint. More information on the surface can be found at: www.physorg.com/news201181250.html .
Astronauts have been shown to have an increased susceptibility to infection while in microgravity, making a deeper understanding of how these bacteria behave in space of particular importance, according to Collins. In addition to its importance in planning future space missions, the research also has important applications here on Earth. The conditions in space are similar to those produced within the human body on several levels. Understanding how bacteria thrive in space may also provide insight into how they develop once they enter the human body.
More information: For additional information on Collins’ research, go to www.rpi.edu/~collic3/Cynthia_Collins
Provided by Rensselaer Polytechnic Institute