Researchers predict material 'denser than diamond'
(PhysOrg.com) -- Stony Brook University graduate student Qiang Zhu, together with Professor of Geosciences and Physics, Artem R. Oganov, postdoc Andriy O. Lyakhov and their colleagues from the University de Oviedo in Spain, have predicted three new forms of carbon, the findings of which were published in a paper entitled "Denser than diamond: Ab initio search for superdense carbon allotropes," in the June 7, 2011 online edition of Physical Review B. So far, each new found modification of carbon resulted in a scientific, technological revolution – the same could happen now, if scientists can find a way to synthesize these new forms of carbon.
Elemental carbon possesses a unique range of structures and properties – from ultrsoft graphite to superhard diamond, and also including elusive carbines, beautifully symmetric fullerenes, carbon nanotubes, and the recently established new form, M-carbon (the structure of which was predicted by Oganov in 2006). Properties of all these modifications of carbon are so interesting and so tunable that two Nobel prizes were awarded recently for their studies (the 1996 Chemistry and 2010 Physics awards).
Graphene is the densest two-dimensional material, with unique mechanical and electronic properties and having some electrons moving with near-light velocities and behaving as if they had zero mass. Diamond has set several records – it is not only the hardest known material, but also has denser packing of atoms than any other known three-dimensional material. When doped by boron, diamond displays superconductivity and is the only know materials simultaneously displaying superhardness and superconductivity.
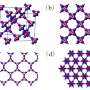
Now Zhu, Oganov, and their colleagues propose three new structures of carbon, which should be more than 3% denser than diamond. Greater density means that electrons should have a higher kinetic energy (that is, move faster). Calculations of Zhu et al. show that the new modifications are almost as hard as diamond, but do not exceed its hardness. Their electronic properties are very diverse, with the band gap ranging from 3.0 eV to 7.3 eV. Band gap is the minimum separation in energy between occupied and unoccupied electronic orbitals and is the most important characteristic of the electronic structure of materials. Such a wide range of band gaps implies the possibility of tuning the electronic properties. The band gap of 7.3 eV predicted for the tP12 modification is the largest value for all forms of carbon.
Other interesting properties include ultralow compressibility – when subjected to pressure, the new forms of carbon will contract less than most materials (even slightly less than diamond, the current record holder). They have higher refractive indices and stronger light dispersion than diamond – which means better brilliance and color effects than those displayed by diamond. “Carbon is an inexhaustible element in its chemical diversity and in the multitude of its physical applications”, says Professor Oganov. “If these predicted forms of carbon can be synthesized, they may find important technological roles”. Researchers believe that the new forms of carbon, thanks to their high densities, could be synthesized by shock compression of low-density modifications, or by directed growth on substrate.
More information: Denser than diamond: Ab initio search for superdense carbon allotropes, Phys. Rev. B 83, 193410 (2011) DOI:10.1103/PhysRevB.83.193410
Abstract
Diamond has the highest number density (i.e., the number of atoms per unit volume) of all known substances and a remarkably high valence electron density (rws = 0.697 Å). Searching for possible superdense carbon allotropes, we have found three structures (hP3, tI12, and tP12) that have significantly greater density. The hP3 and tP12 phases have strong analogy with two polymorphs of silica (β-quartz and keatite), while the tI12 phase is related to the high-pressure SiS2 polymorph. Furthermore, we found a collection of other superdense structures based on the motifs of the aforementioned structures, but with different ways of packing carbon tetrahedra, and among these the hP3 and tI12 structures are the densest. At ambient conditions, the hP3 phase is a semiconductor with the GW band gap of 3.0 eV, tI12 is an insulator with the band gap of 5.5 eV, while tP12 is an insulator, the band gap of which is remarkably high (7.3 eV), making it the widest-gap carbon allotrope. These allotropes are metastable and have comparable to diamond or slightly higher bulk moduli; their Vickers hardnesses are calculated to be 87.6 GPa for hP3, 87.2 GPa for tI12, and 88.3 GPa for tP12, respectively, thus making these allotropes nearly as hard as diamond (for which the same model gives the hardness of 94.3 GPa). Superdense carbon allotropes are predicted to have remarkably high refractive indices and strong dispersion of light.
Provided by Stony Brook University