A chaperone system guides tail-anchored membrane proteins to their destined membrane
Newly synthesized proteins can only fold into their correct three dimensional structure thanks to chaperones. In case of membrane proteins chaperones do not only prevent their aggregation, but also escort them to their destination and aid in membrane insertion. The underlying molecular mechanism has now been resolved for tail-anchored membrane proteins.
A newly synthesized protein is as fragile as a newborn baby. It could never fold into its correct three dimensional structure if it was not protected by chaperones within the densely populated cytosol. In case of membrane proteins chaperones do not only pre-vent their aggregation, but also escort them to their destination and aid in membrane insertion. The underlying molecular mechanism of how a certain family of membrane proteins is targeted and inserted into membranes has now been resolved by an international research team with participation of the Goethe University Frankfurt. These proteins are anchored within the membrane via a single helix and thus are called "tail-anchored" (TA) proteins.
The key for proper protein sorting are signal sequences which are decoded by chaperones. As soon as they - together with their "foster child" - arrive at their destination the interaction with a specific receptor within the target membrane initiates membrane insertion. Protein components responsible for the insertion of TA proteins have recently been identified. The molecular mechanisms of how these sorting systems work, however, were not known so far.
In this interdisciplinary study, that will be released in the current "online" issue of "Science", the research groups of Prof.Volker Dötsch (Goethe University Frankfurt), Prof. Irmgard Sinning (Heidelberg University Biochemistry Center) and Prof. Vlad Denic (Harvard University (USA)) were able to solve the question by a combination of diverse methods such as X-Ray Crystallography and NMR-Spectroscopy as well as biochemical and cell-biological approaches.
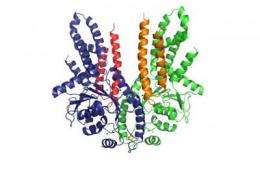
In detailed biophysical studies Volker Dötsch´s research group showed that the central cha-peron of the responsible protein complex, called Get3, regulates both binding to TA proteins within the cytosol and their release at the membrane. The two receptor proteins Get1 and Get2 aid in TA protein insertion. They use overlapping interfaces for the interaction with the Get3 ATPase. On the basis of different crystal structures the researchers suggest a model for the mechanism of how TA proteins are inserted into the membrane. Upon interaction with its membrane receptor the Get3 dimer gradually opens up to allow for the controlled TA protein insertion. "Those results are particularly important because they enabled us to establish the first model of the receptor-assisted membrane insertion of TA proteins that will now be the basis for further studies" comments Dötsch.
More information: Stefer, S. et al: Structural basis for tail-anchored membrane protein biogenesis by the Get3-receptor complex, Science (2011), DOI: 10.1126/science.1207125
Provided by Goethe University Frankfurt

















