'Imprinted' developmental genes gain new roles in adult stem cells
(PhysOrg.com) -- The repair of tissues damaged by injury or illness relies on the ability of adult stem cells to grow and self-renew. But this ability needs to be tightly controlled; if regulation is lost, the stem cells may instead give rise to cancer. A study from Children’s Hospital Boston finds that a network of genes crucial in embryonic development may also keep tight rein on adult stem cells in the lung and other tissues, particularly as these cells rally to repair tissue damage.
The findings are the first to link this set of genes, called an “imprinted gene network,” to tissue repair, and suggest that these genes may play fundamental biological roles in maintaining the “stemness” of adult stem cells.
A team of researchers led by Carla Kim, PhD, of the Stem Cell Research Program at Children's Hospital Boston, reported the discovery in the September 2 issue of the journal Cell Stem Cell.
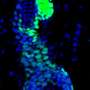
Kim’s team studied renewal of stem cells in the lung (called bronchoalveolar stem cells, or BASCs) and the influence of a protein called Bmi1, known to regulate adult stem cell function and tumor cell development in many organs. “Bmi1 is required for many kinds of adult stem cells to renew themselves,” Kim explained, “and its expression is an essential factor in some cancers, including lung tumors.”
Her laboratory made use of a mouse previously engineered to lack Bmi1 to study what happens after lung injury. “We were surprised to find that the loss of Bmi1 lead to overexpression of a network of imprinted genes in lung stem cells,” Kim said.
Every person’s genome harbors two copies of every gene, one from their mother and one from their father. Generally, our cells use both copies of every gene equally, but in the case of “imprinted” genes (which constitute a small percentage of our genome), our cells only use one copy and silence the second.
The network that Kim and her team studied comprises 14 imprinted genes that are all active in certain tissues during embryonic development. The activity of these genes – each of which individually impacts other pathways – dials down as we reach adulthood.
To understand their importance in BASC function, Kim’s team measured their expression in a model of lung injury.
In normal lung stem cells, the expression (activity) of imprinted genes, in particular a gene named p57, decreased shortly after injury, peaked a few days later, and dropped back to baseline levels once repair was complete. In lung cells lacking Bmi1, expression of imprinted genes remained high even weeks after injury.
“In normal BASCs, the pattern of expression suggests that Bmi1 and this imprinted gene network make sure that when the lung stem cells are called in to repair an injury, they stop when the repair is complete,” Kim said. “This is the first time anyone has found a link between imprinted genes and tissue repair.”
Kim believes that Bmi1 acts as a second layer of control that fine-tunes the expression of imprinted genes. “We think Bmi1 helps make sure the active copies of these imprinted genes are only expressed just enough, and are turned off when the stem cell needs them to be completely silent,” she explained. “If the imprinted genes are expressed too much or too little, the lung stem cells can’t self-renew.”
The findings also reveal a hitherto unknown role for imprinted genes in regulating the growth of adult stem cells from the lung and potentially other tissues, adding them to the list of “stemness factors” that help stem cells maintain their unique capabilities. Kim also believes that these imprinted genes provide an opportunity to look for new pathways involved in stem cells’ response to injury.
More information: www.sciencedirect.com/science/ … ii/S1934590911003377
Provided by Children's Hospital Boston