Woolly mammoth's secrets for shrugging off cold points toward new artificial blood for humans
The blood from woolly mammoths -- those extinct elephant-like creatures that roamed the Earth in pre-historic times -- is helping scientists develop new blood products for modern medical procedures that involve reducing patients' body temperature. The report appears in ACS' journal Biochemistry.
Chien Ho and colleagues note that woolly mammoth ancestors initially evolved in warm climates, where African and Asian elephants live now, but migrated to the cold regions of Eurasia 1.2 million – 2.0 million years ago in the Pleistocene ice age. They adapted to their new environment by growing thick, "woolly" fur and smaller ears, which helped conserve heat, and possibly by changing their DNA. In previous research, Ho and colleagues discovered that a blood protein (hemoglobin) that carries oxygen from the lungs to the rest of the body in the woolly mammoth has mutations in its DNA that make it different from that of its cousin, the Asian elephant. The scientists turned to the mutations that helped woolly mammoths survive freezing temperatures, and carefully analyzed hemoglobin from the ancient animal.
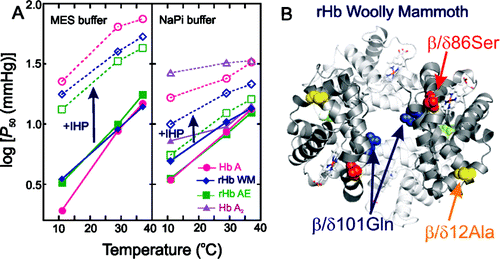
They didn't have a woolly mammoth blood sample, so they made the hemoglobin protein in the laboratory by using fragmented DNA sequences from three mammoths that died in Siberia between 25,000 and 43,000 years ago. Compared to hemoglobin from Asian elephants and humans, the woolly mammoth protein was much less sensitive to temperature changes, which means it can still easily unload oxygen to tissues that need it in the cold, whereas the other hemoglobins can't. This is likely due to at least two of the mutations in the woolly mammoth hemoglobin gene. These insights could lead to the design of new artificial blood products for use in hypothermia induced during heart and brain surgeries.
More information:
A Biochemical–Biophysical Study of Hemoglobins from Woolly Mammoth, Asian Elephant, and Humans, Biochemistry, 2011, 50 (34), pp 7350–7360
DOI: 10.1021/bi200777j
Abstract
This study is aimed at investigating the molecular basis of environmental adaptation of woolly mammoth hemoglobin (Hb) to the harsh thermal conditions of the Pleistocene ice ages. To this end, we have carried out a comparative biochemical–biophysical characterization of the structural and functional properties of recombinant hemoglobins (rHb) from woolly mammoth (rHb WM) and Asian elephant (rHb AE) in relation to human hemoglobins Hb A and Hb A2 (a minor component of human blood). We have obtained oxygen equilibrium curves and calculated O2 affinities, Bohr effects, and the apparent heat of oxygenation (ΔH) in the presence and absence of allosteric effectors [inorganic phosphate and inositol hexaphosphate (IHP)]. Here, we show that the four Hbs exhibit distinct structural properties and respond differently to allosteric effectors. In addition, the apparent heat of oxygenation (ΔH) for rHb WM is less negative than that of rHb AE, especially in phosphate buffer and the presence of IHP, suggesting that the oxygen affinity of mammoth blood was also less sensitive to temperature change. Finally, 1H NMR spectroscopy data indicates that both α1(β/δ)1 and α1(β/δ)2 interfaces in rHb WM and rHb AE are perturbed, whereas only the α1δ1 interface in Hb A2 is perturbed compared to that in Hb A. The distinct structural and functional features of rHb WM presumably facilitated woolly mammoth survival in the Arctic environment.
Provided by American Chemical Society