October 24, 2011 feature
Decoding early Martian weather: Analyzing carbonate minerals in meteorite Allan Hills 84001
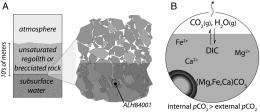
(PhysOrg.com) -- While geological evidence points to the presence of liquid water on Mars during the Noachian epoch (the period from 4.5 to 3.5 billion years ago), determining the temperature of that water – a factor critical to the probability of its ability to support early life – has hitherto been impossible. Recently, however, researchers at California Institute of Technology Geological and Planetary Sciences have derived a precipitation temperature of 18 °C from carbonate minerals found in the 4.1 billion-year-old Allan Hills 84001 (ALH84001) meteorite. Although this ancient aquifer’s temperature was relatively mild, the researchers note that their findings do not necessarily demonstrate habitability.
Led by Itay Halevy (currently at the Weizmann Institute of Science in Israel), along with Woodward W. Fischer and John M. Eiler, the research team faced a number of challenges. “The main challenge,” Halevy says, “was making an isotope clumping measurement on a precious, low-carbonate-abundance material.” Isotope clumping in carbonates denotes the tendency of heavy carbon and oxygen isotopes in carbonates (and in CO2) to bond with each other rather than with the elements’ lighter isotopes. This temperature-dependent tendency is the basis of multiply substituted (clumped) isotope thermometry.
Clumped isotope thermometry is based on the tendency of heavy carbon and oxygen isotopes to bond with each other rather than with the lighter isotopes of these elements in a way that is effectively independent of the material's isotopic composition: However abundant or rare the heavy isotopes are in a sample, they will still prefer to form bonds with each other, and this preference will always depend on temperature – and the colder it is, the higher the preference. “Other isotopic thermometers are based on the temperature dependence of the difference in chemical or isotopic composition between the aqueous solution and the carbonates that precipitate from it,” notes Halevy, “so to derive temperature one must know the chemical or isotopic composition of both the carbonates and the parent fluid. Unfortunately, it is impossible to know the composition of ancient fluids and so one must make some assumptions about their composition. Any temperature determined in this way is always dependent on these assumptions.” Clumped isotope thermometry avoids this because no matter how abundant the heavy isotopes are, they will always tend to bond in a temperature-dependent way.
“The clumping measurement, developed by John Eiler and his students and postdocs at Caltech over the last seven to eight years, is itself challenging to make,” Halevy continues. The natural abundance of C-O clumps is only a few tens parts-per-million, which means you have to measure a lot of material to get a precise and accurate result – and we didn’t have a lot of material. ALH84001 is a 4-billion-year-old, originally 2 kg Martian rock; we had 5.5 grams, using 3 grams in the analysis – and of these 3 grams, only about 1% was carbonate minerals.”
While that was an appreciable amount of carbonate for a single clumping measurement, the researchers wanted to cut the gas into three measurements to get an internal stratigraphy (the study of strata, or layers) of the carbonate concretions. “To this,” Halevy comments, “add the increased risk of sample contamination associated with long acid digestion times – up to 12 hours for the more recalcitrant magnesium-rich carbonates – and you have the makings of an analytical challenge.”
To overcome the low abundance of carbonate, the team performed a microvolume measurement. “Basically,” Halevy explains, “we froze the sample of CO2 gas released from the carbonates by acid digestion into a very small volume, shut it off from a lot of dead volume in the plumbing of the mass spectrometer, and thawed it in the small volume alone. The high pressure achieved by compressing the gas into a small volume allowed us to make the measurement under conditions that are close to the way the clumping measurement is typically made. Still, there are some important differences from a typical clumping measurement, so it took a lot of experimentation to map how the microvolume affected the results and to figure out ways to minimize these effects – or at least account for them.” As for the increased risk of contamination, the team performed extra purification steps to remove miscellaneous contaminants and again experimented extensively to convince themselves that the measurement was in no way compromised by the long reaction times.
There are other innovations that could be applied to the protocol, mainly having to do with instrument improvements. “As mass spectrometers become more sensitive and are able to better differentiate between molecules of slightly differing mass,” Halevy explains, “low sample abundance and contamination will cease to be issues. A clumping measurement would be easier to make on small amounts of sample and any contaminations would be easily distinguishable from the CO2 molecules of interest. This would increase confidence in the measured temperature. “
Halevy also points out that they developed the aquifer hypothesis on the basis of isotopic variability within the carbonate concretions and in light of the new insight that the temperature hovered around 20 °C. “Basically, given the temperature, the isotopic variability can only be explained by the drying-out of an aqueous reservoir that did not exchange CO2 freely with the atmosphere. The only environment we could come up with that meets these requirements is a subsurface aquifer. Increased confidence in the measured temperature would translate into increased confidence in this model for the formation environment of the carbonates.”
Regarding computer modeling, Halevy says that while computer models are important for developing a quantitative understanding of analytical results, such as those presented in the paper, they can't replace the measurements. “The measurements provide the physical constraints,” Halevy states, “and we can then use computer simulation to try and tease out information about process and environment.” In the team’s study, for example, measurements yielded the temperature and isotopic composition of the aqueous solution, while computer simulations allowed a quantitative estimate of how much of the water had to have evaporated, depending on the conditions of evaporation.
In terms of applying their findings to exoplanetary exploration, Halevy stresses that “The mass spectrometers on or rovers, as well as the means for sample preparation and purification, would have to improve dramatically for a carbonate clumping measurement to be made in situ. Nevertheless, this may not be beyond the realm of possibility and may teach us much about aqueous processes on other planets. In addition, the ability to make a carbonate clumping measurement automatically implies the ability to measure clumping in CO2 and, therefore, the ability to explore certain atmospheric processes. Finally, clumping in molecules other than CO2 – such as organic molecules – may hold information not only about temperature, but the processes that formed them as well.”
Halevy’s research will continue to include an analytical/experimental component and a modeling/simulation component. “Providing quantitative constraints on chemical processes on modern and ancient Mars will continue to feature prominently in my research,” Halevy concludes. “These same approaches will also be applied to understanding the geochemistry of Earth's early oceans and atmospheric evolution.”
More information: Carbonates in the Martian meteorite Allan Hills 84001 formed at 18 ± 4 °C in a near-surface aqueous environment, Published online before print October 3, 2011, PNAS October 11, 2011 vol. 108 no. 41 16895-16899, doi:10.1073/pnas.1109444108
Copyright 2011 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.





















