November 11, 2011 report
Researchers moving closer to a soluble solution to Haber-Bocsh process
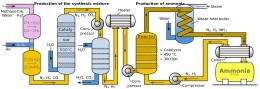
(PhysOrg.com) -- The Haber-Bosch process, known throughout the world as the means by which ammonia is made for use in fertilizer, has been under study for at least as long as the agricultural revolution has been underway. While the current system clearly works, it’s been used to feed the billions of people on the planet for the past several decades; it’s also costly due to the high temperature and pressure involved. If a way could be found to produce the same result at non-elevated temperatures, the production costs would come down dramatically resulting in reduced food prices the world over. No small thing considering we just welcomed the seven billionth person just last week. The good news is that some progress is being made. Patrick Holland and his colleagues at the University of Rochester, and at the Max Planck Institute in Germany, describe in their paper published in Science, how they have been making inroads into using a soluble iron compound to promote the process.
As all those who have taken very many chemistry courses know, the Haber-Bosch process involves heating a potassium-doped iron catalyst under high pressure, then mixing it over with hydrogen gas to generate the end product, ammonia.
What Holland and his team are trying to do is recreate the same process using a different catalyst. Instead of the regular iron catalysts used in current processes, they would like to use a soluble iron catalyst, which most agree would allow for the process to work under room temperatures. Unfortunately, work by others in trying the same thing has thus far resulted in less than dramatic results, i.e. not enough ammonia was produced.
This new research however looks more promising. What the team has done so far is develop a new iron material that will react with nitrogen gas when exposed to potassium which generates a material that has two nitrides which contain a mixed iron nitride core - which will react with hydrogen gas to create a reasonably large amount of ammonia.
While this doesn’t exactly solve the puzzle of how to get the H-B process to work at room temperature and at normal pressure (because the iron is consumed, thus it’s not truly catalytic) it is a step in the right direction, and the team is optimistic that because of knowledge gained in their experiments, they will be able to build a complex that is truly catalytic, which will then lead to a real solution to the underlying problem.
More information:
N2 Reduction and Hydrogenation to Ammonia by a Molecular Iron-Potassium Complex, Science 11 November 2011:
Vol. 334 no. 6057 pp. 780-783. DOI: 10.1126/science.1211906
ABSTRACT
The most common catalyst in the Haber-Bosch process for the hydrogenation of dinitrogen (N2) to ammonia (NH3) is an iron surface promoted with potassium cations (K+), but soluble iron complexes have neither reduced the N-N bond of N2 to nitride (N3–) nor produced large amounts of NH3 from N2. We report a molecular iron complex that reacts with N2 and a potassium reductant to give a complex with two nitrides, which are bound to iron and potassium cations. The product has a Fe3N2 core, implying that three iron atoms cooperate to break the N-N triple bond through a six-electron reduction. The nitride complex reacts with acid and with H2 to give substantial yields of N2-derived ammonia. These reactions, although not yet catalytic, give structural and spectroscopic insight into N2 cleavage and N-H bond-forming reactions of iron.
Journal information: Science
© 2011 PhysOrg.com