November 15, 2011 report
Pair claim they have turned hydrogen to metal
(PhysOrg.com) -- Many have tried, but none have succeeded. For at least a hundred years, scientists looking at hydrogen have scratched their chins when musing over the fact that it, as an alkali metal, by all rights should exist as a metal under the right circumstances. But thus far, no one has been able to figure out what the right circumstances might be. Until now. Maybe. Mikhail Eremets and Ivan Troyan of the Max-Planck Institute describe in their paper published in Nature Materials, how they subjected a sample of hydrogen to high pressure and low temperature and found it then demonstrated properties generally ascribed to a metal.
One of the problems in attempting to say whether something is a metal or not, is the somewhat flimsy criteria used to describe just what exactly a metal is. Most dictionaries describe it loosely as an electropositive element that probably should be shiny, a good conductor of both heat and electricity and should be malleable to some degree. And of course, common sense says that it probably ought to be solid at some temperature or pressure. And that’s the crux of the matter in trying to get hydrogen to look and act like a solid. All manner of people have subjected it to either or both and have failed to produce anything that most would say is a metal and that’s why this latest attempt by Eremets and Troyan has met with less than wild enthusiasm in the science world.
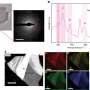
Regardless, they say that when they put a sample of hydrogen in a alumina-epoxy gasket that they put inside of a diamond anvil cell, an arrangement that allowed them to test the opacity via laser and the electrical resistance using electrodes, they found that without heating or cooling and at a pressure of 220GPa, the sample clouded to the point of becoming opaque and began to demonstrate an ability to conduct electricity. Next, the temperature was lowered to 30K and the pressure increased to 260GPa where they found an electrical resistance increase of 20 percent, before it leveled off. This the team says, shows the sample displaying metallic attributes.
Others will of course have to duplicate the process and find the same results, and if they do, then discussions will likely ensue among the scientific community to determine if what was observed can truly be used to claim that the process does indeed turn hydrogen into a metal.
On the other hand the whole point might be made moot by the simple fact that the procedure clearly can make hydrogen conductive at room temperature, which means it could conceivably turn out to be that elusive superconductor that scientists the world over have been searching for.
More information: Conductive dense hydrogen, Nature Materials (2011) doi:10.1038/nmat3175
Abstract
Molecular hydrogen is expected to exhibit metallic properties under megabar pressures. This metal is predicted to be superconducting with a very high critical temperature, Tc, of 200–400 K, and it may acquire a new quantum state as a metallic superfluid and a superconducting superfluid2. It may potentially be recovered metastably at ambient pressures. However, experiments carried out at low temperatures, T<100 K, showed that at record pressures of 300 GPa, hydrogen remains in the molecular insulating state. Here we report on the transformation of normal molecular hydrogen at room temperature (295 K) to a conductive and metallic state. At 200 GPa the Raman frequency of the molecular vibron strongly decreased and the spectral width increased, evidencing a strong interaction between molecules. Deuterium behaved similarly. Above 220 GPa, hydrogen became opaque and electrically conductive. At 260–270 GPa, hydrogen transformed into a metal as the conductance of hydrogen sharply increased and changed little on further pressurizing up to 300 GPa or cooling to at least 30 K; and the sample reflected light well. The metallic phase transformed back at 295 K into molecular hydrogen at 200 GPa. This significant hysteresis indicates that the transformation of molecular hydrogen into a metal is accompanied by a first-order structural transition presumably into a monatomic liquid state. Our findings open an avenue for detailed and comprehensive studies of metallic hydrogen.
via RCS
Journal information: Nature Materials
© 2011 PhysOrg.com