Advance toward an imaging agent for diagnosing Alzheimer's disease
Scientists are reporting development and initial laboratory tests of an imaging agent that shows promise for detecting the tell-tale signs of Alzheimer's disease (AD) in the brain — signs that now can't confirm a diagnosis until after patients have died. Their report appears in the journal ACS Medicinal Chemistry Letters.
Masahiro Ono and colleagues explain that no proven laboratory test or medical scan now exists for AD, which is claiming an increasingly heavy toll with the graying of the world's population. Patients now get a diagnosis of AD based on their medical history and symptoms, and symptoms like memory loss often are identical to those of normal aging. Currently, the only definitive way to diagnose AD involves an autopsy with examination of brain samples for the presence of the clumps and tangles of abnormal protein that occur in the disease.
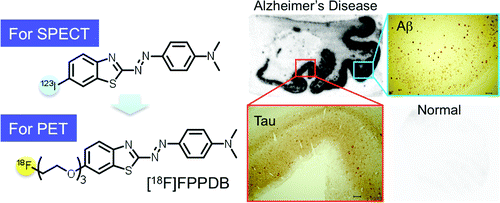
The scientists describe the synthesis and lab testing of a new imaging agent (called FPPDB), which bound tightly to ß-amyloid plaques and neurofibrillary tangles — signs of AD — in human brain samples. In normal laboratory mice, which served as stand-ins for humans, FPPDB stayed in the body long enough for a PET scan (a sophisticated medical imaging technique). With further development, the imaging agent may allow early AD diagnosis in humans, the scientists indicate.
More information: 18F-Labeled Phenyldiazenyl Benzothiazole for in Vivo Imaging of Neurofibrillary Tangles in Alzheimer's Disease Brains, ACS Med. Chem. Lett., Article ASAP. DOI: 10.1021/ml200230e
Abstract
We synthesized and evaluated (E)-4-((6-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)benzo[d]thiazol-2-yl)diazenyl)-N,N-dimethylaniline (FPPDB) as a probe for the imaging of neurofibrillary tangles (NFTs) in patients with Alzheimer's disease (AD). In assays using thioflavin S (ThS) as a competitive ligand, FPPDB competed with ThS well and showed high affinity for both tau and Aβ1–42 aggregates (Ki = 13.0 and 20.0 nM, respectively). The results of saturation binding assays also verified that FPPDB bound to both tau and Aβ1–42 aggregates with high affinity (Kd = 44.8 nM and Bmax = 45.8 pmol/nmol protein for tau aggregates and Kd = 45.4 nM and Bmax = 38.9 pmol/nmol protein for Aβ1–42 aggregates). Furthermore, [18F]FPPDB substantially labeled NFTs and senile plaques in AD brain sections but not control brain sections. In biodistribution experiments using normal mice, [18F]FPPDB displayed higher uptake (4.28% ID/g at 2 min postinjection) into and washout (2.53% ID/g at 60 min postinjection) from the brain with time. On the basis of the chemical structure of FPPDB, further increases in selective binding to tau aggregates may lead to the development of more useful probes for the imaging of NFTs in AD brains.
Journal information: ACS Medicinal Chemistry Letters
Provided by American Chemical Society