January 26, 2012 feature
Of microchemistry and molecules: Electronic microfluidic device synthesizes biocompatible probes
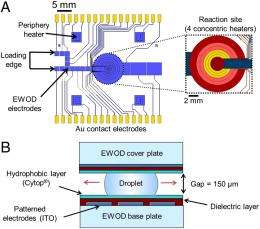
(PhysOrg.com) -- Microfluidic chemistry is fast gaining popularity – and for good reason: In addition to allowing highly-precise reaction control, micro-reactions often exhibit higher yield and proceed faster than their macroscale cousins. They also readily scale to production environments, and are far safer when synthesizing hazardous compounds because of the very small volumes of materials inside the devices. Recently, scientists at the University of California, Los Angeles (UCLA) have advanced the field by developing and demonstrating an all-electronic digital microfluidic device for microscale chemical synthesis in organic solvents – and that is operated by electrowetting-on-dielectric, or EWOD. (Electrowetting modifies the wetting properties of a surface by applying an electric field; EWOD coats the electrode forming that surface with a dielectric insulating layer.) Their robust EWOD platform simultaneously resolves two limitations of previous technologies – namely, multistep reaction protocols and organic solvent compatibility.
The research team, led by Professors R. Michael van Dam and Pei Yuin Keng in UCLA’s Crump Institute for Molecular Imaging and Professor CJ Kim in the Mechanical and Aerospace Engineering Department, faced a particularly challenging issue in developing their digital microfluidic device and applying it to microscale chemical synthesis. “When working with organic solvents at small volume scales – especially those that are volatile – evaporation is a significant problem in the relatively open configuration of EWOD chips,” van Dam tells PhysOrg.com. “Unwanted evaporation can change concentrations, dry the sample, and so on, leading to imprecise control over the chemical process and low reproducibility of the chemistry. Our main challenge was in overcoming this effect.”
Controlling liquids via electrowetting is very attractive due to the absence of moving parts, as well as the ease of integrating droplet actuation with heating and sensing. “It had been shown a few years ago that organic solvents can be manipulated on the same chips as water droplets, although not exactly by pure electrowetting,” van Dam continues. “Indeed, we didn’t have any problems moving droplets. Rather, the main operational challenges we encountered were related to on-chip mixing of liquids with solid residues, and the well-controlled evaporation of solvents at temperatures above the solvent boiling point.” This is due to the fact that under such superheated conditions, liquids can undergo bumping – the bursting of droplets and loss of reagents out of the chip.
The team explored a number of ideas to address the issue of undesired evaporation during reaction steps. “Altering the chip and droplet geometry to limit evaporation was somewhat effective, but also adversely impacted the ability to evaporate solvents during steps where evaporation was actually desired,” van Dam explains. Replenishing the solvent by loading additional droplets is a promising approach – but other technical challenges would then need to be addressed, such as how to avoid a drop in reaction temperature when a new droplet is added, and how to effectively mix the incoming droplet with the existing reaction mixture.
“The real breakthrough in the application we presented was realizing that we could alter the solvent without encountering the same difficulties associated with doing so at the macroscale. Since our reaction volume is so small, we could effectively evaporate dimethyl sulfoxide (DMSO) – a very non-volatile solvent – at a modest temperature.” At the macroscale, DMSO is typically avoided because it is very difficult to remove quickly – a concern in certain applications where synthesis time is critical, such as synthesis of positron emission tomography (PET) probes – and more volatile solvents are selected instead. However, van Dam points out, for some applications, the length of time would be less critical – for example, chemical and pharmaceutical production processes can take days, weeks or months.
Moreover, adds van Dam, the team is developing several additional innovations to enhance the current experimental design. “One area we’re working on is increasing the level of automation,” van Dam illustrates. “Once the droplets of chemicals are on-chip, they’re manipulated electronically, so sophisticated sequences of operations can readily be automated. In contrast,” he continues, “in our proof-of-concept synthesis chip, the necessary steps of adding reagents to the chip and extracting the final product are performed by pipetting or other manually-operated techniques. Increased overall automation is therefore critical to making the platform user-friendly and safe.
Another area of investigation is in situ sensing of liquid droplets. “With a very simple modification of the EWOD voltage driving circuit, it’s possible to monitor the AC current through the droplet, says van Dam. “This small current gives information about impedance, which in turn is related to droplet volume and composition. Other groups have shown how verifying that droplets have actually moved as instructed can increase the fidelity of on-chip assays and we’re planning to extend this principle to verify that the correct liquid is in the correct location and to perform real-time monitoring of chemical process variables.” This could be used, for example, to increase the reliability of on-chip microchemical production or provide an integrated readout for a chemical assay.
Van Dam also notes that microscale will eventually transition to nanoscale. “There are research efforts underway to shrink the size of electrodes and droplets handled by EWOD microfluidic devices to subnanoliter volumes. The microchemistry principles we presented could likely be scaled down to operate on these devices.”
On the other hand, van Dam points out that an in silico simulation model would be difficult to derive, given the current general lack of understanding of microscale chemistry in droplets. “For example,” he relates to PhysOrg, “one surprising result we observed was the need to use somewhat higher reagent concentrations in droplets compared to what is normally used at the macroscale to achieve comparable reaction yields. Our microchemistry platform could perhaps be used to study microscale chemistry and gather data that could lead to development of a simulation.”
One of the next steps in the group’s work is to increase the level of automation as mentioned above. “We envision a compact, benchtop system that, if loaded with the right reagents, could produce a variety of compounds on demand at the push of a button. We’re also pursuing applications of this device – in particular for the production of PET probes. Unlike most chemicals which can be produced in large batches and stored, these compounds are short-lived and require production just prior to use for medical imaging.” Currently, the production of PET probes requires expensive, complicated, and bulky equipment and infrastructure.
“Commercial networks of radiopharmacies have invested in this equipment and produce and ship the probes daily to supply hospitals, imaging centers, and research labs,” van Dam adds. “Making large batches that are divided among numerous customers provides economy of scale that makes these probes affordable, but at a cost – these radiopharmacies provide only a small number of different probes. But as we move into an era of personalized medicine, it will become increasingly important to have a diversity of diagnostic probes available so that patients can be matched to the correct drugs.” Compact, inexpensive, benchtop chemistry systems could be transformative, in that clinicians and researchers could afford to produce exactly the probes they want, when they want.
Van Dam also points out that although these techniques have been demonstrated in the context of medical diagnostics, many different areas could also be served by performing microchemistry on EWOD. “Small scale chemistry could be useful to chemists doing natural products synthesis, where reagents and intermediates can be very costly due to the large number of reaction steps, and time, needed to produce them,” he adds. “It’s also likely that the ability to handle chemicals and organic solvents on-chip could lead to new assays in a variety of areas such as contaminant detection, environmental monitoring, and quality control in chemical production.” The techniques might also be useful for optofluidics due to the use of organic and high-index liquids in such devices.
“EWOD chips enable programmable control of liquids and thus a single chip design may be capable of supporting a wide range of reactions and assays with only software changes,” van Dam concludes. “By not having to produce a different chip for each application, cost could be substantially reduced.”
More information: Micro-chemical synthesis of molecular probes on an electronic microfluidic device. PNAS January 17, 2012 vol. 109 no. 3 690-695, doi: 10.1073/pnas.1117566109
Journal information: Proceedings of the National Academy of Sciences
Copyright 2012 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.


















