Big, bad bacterium is an 'iron pirate'
(PhysOrg.com) -- Life inside the human body sometimes looks like life on the high seas in the 1600s, when pirates hijacked foreign vessels in search of precious metals.
For Neisseria bacteria, which can cause gonorrhea and meningitis, the booty is not gold or silver but plain old iron.
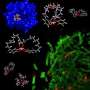
Until recently, scientists did not understand how these bacteria snatch iron from healthy human cells, where a protein called transferrin binds the metal in a molecular bear-hug. However, a new study led by scientists at the National Institutes of Health in collaboration with biophysicist James Gumbart at the U.S. Department of Energy's Argonne National Laboratory has demonstrated the likely process by which the bacteria steal the biologically valuable metal.
Within the pathogenic Neisseria bacteria, the iron transport system consists of a two-part membrane protein complex that binds human transferrin. At the molecular level, the primary membrane protein involved—called TbpA—resembles a narrow barrel, woven from a strand of amino acids, the building blocks of all proteins. When not in the process of transport, the barrel of TbpA is blocked by a separate region of the same protein that forms a kind of "plug" to prevent other molecules from freely entering or leaving the bacterium, said Gumbart.
"Normally, this protein looks something like a wine bottle with a cork inside of it," he said. "When an iron-containing transferrin comes along though, TbpA is opened by another protein inside the bacterium, which pulls on the cork and brings the iron in."
To gain a better understanding of how the theft unfolds in real time, Gumbart developed computational models that highlight electrostatic changes in TbpA during opening. Initially, the interior of the barrel is negatively charged, creating a "sucking" effect that extracts the iron from transferrin. As TbpA opens, however, the electrostatic potential gradient in the barrel becomes more positive, thereby expelling the iron into the bacterial cell.
The long-term goal of the research, according to Gumbart, is to use this discovery to drive the development of a new class of antibiotics that would prevent the heists from taking place.
"Without iron, these bacteria don't have a hope of surviving," he said.
"Proteins like TbpA make highly attractive targets because they are unique to specific classes of bacteria," Gumbart added.
"Now that we have a better idea of how this process works, we should be able to use the knowledge we've gained to combat these diseases."
The result of the study appears online in the journal Nature.
Provided by Argonne National Laboratory