Structural and functional secrets of a zinc transporter protein with a role in human disease
Up to one-tenth of the proteins encoded in the human genome incorporate zinc, making this element indispensable to biological function. Indeed, most organisms employ a host of specialized transporter proteins to maintain appropriate zinc levels, and failures in these pathways can cause serious health issues.
Recent research from Toshiyuki Fukada and colleagues at the RIKEN Research Center for Allergy and Immunology in Yokohama has characterized the structure and function of one of these proteins, ZIP13.1 From previous research on ZIP13, they knew that mice lacking this protein suffer diverse problems2. “Zinc-related events modulated bone, tooth and connective tissue development,” says Fukada, “and there was a link between ZIP13 and human disease.” For example, patients with a new type of inherited disease, currently called spondylocheiro dysplastic Ehlers-Danlos syndrome (SCD-EDS) that mirrors defects seen in these mice, exhibit a crippling mutation in this gene.
ZIP13 belongs to a subset of proteins that facilitate zinc entry into the cell, known as the LIV-1 subfamily of ZIP zinc transporters (LZT)3. From their characterization work, Fukada and colleagues confirmed that increased ZIP13 expression leads to more efficient zinc influx.
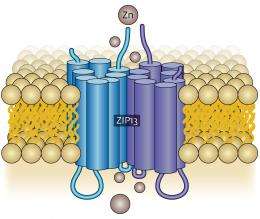
Intriguingly, ZIP13 resides within the membranes of the Golgi apparatus, an organelle responsible for protein processing, rather than at the outer membrane of the cell. This suggests that ZIP13 may manage intracellular zinc levels by directing the release of zinc from the Golgi.
Fukada and colleagues also discovered that ZIP13 features a largely unstructured loop domain that extends outside the membrane. Although previous studies have linked this region with protein degradation in other LZT proteins, it appears to play no such role for ZIP13. The researchers were also surprised to find that ZIP13 molecules act in pairs, forming structures known as homo-dimers (Fig. 1). Fukada speculates that this may offer important clues into this protein’s function.
“The mechanism of zinc transport used by ZIP13 and other LZT proteins remains unknown. This is an open question,” says Fukada. Further progress will require investigation of the atomic-level structure of ZIP13 and related proteins.
He hopes that their work on ZIP13 will benefit patients with conditions associated with abnormal zinc regulation. Defining ZIP13 function will also be a major step towards understanding the mechanisms that determine each zinc transporter’s biological specificity, and how they contribute to cellular functions. “One of my general interests in ‘zinc biology’ is to understand the logic behind how each zinc transporter pathway specifically controls biological events,” says Fukada.
More information: 1. Bin, B.H., et al. Biochemical characterization of human ZIP13 protein: a homo-dimerized zinc transporter involved in the Spondylocheiro dysplastic Ehlers-Danlos syndrome. Journal of Biological Chemistry 286, 40255–40265 (2011).
2. Fukada, T., et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS ONE 3, e3642 (2008).
3. Fukada, T. & Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 3, 662–674 (2011).
Provided by RIKEN