Toppling Raman shift in supercritical carbon dioxide
(PhysOrg.com) -- Just as a wine glass vibrates and sometimes breaks when a diva sings the right note, carbon dioxide vibrates when light or heat serenades it. When it does, carbon dioxide exhibits a vibrational puzzle known as Fermi resonance. Now, researchers studying geologic carbon storage have learned a bit more about the nature of carbon dioxide.
The results provide clues to the nature of the Fermi resonance in other molecules, and will help researchers better understand details in chemical reactions. The team of researchers from the Department of Energy's Pacific Northwest National Laboratory report their findings in the February 28 issue of the journal in Physical Chemistry Chemical Physics.
"We're happy to be able to say something new about something so old," said PNNL chemist and author Charles Windisch, Jr. "We figured out how the different carbon dioxide molecules are vibrating at some of the Fermi resonance frequencies. And, of course, we can calibrate our data with more accuracy now."
"Even to this day, people mark Raman spectra incorrectly," said PNNL computational chemist Vassiliki-Alexandra Glezakou. "It helps to know what we are looking at, if we are going to use certain bands as guidelines to understand molecular interactions."
Carbon Dioxide Conundrum
The PNNL researchers did not set out to study again a phenomenon that dates back to the 1930s. Instead, they wanted to investigate what happens when carbon dioxide is stored underground as part of a national research effort to reduce carbon emissions from power generation. To do so, researchers plan to inject carbon dioxide in an unusual form of the gas that behaves like a liquid due to being under high pressure, called supercritical. To follow supercritical carbon dioxide in chemical reactions, researchers often use a technique called Raman spectroscopy.
Raman spectroscopy is a way of capturing a molecule's vibration. Simple molecules can vibrate in well-defined modes such as stretching and bending, which correspond to peak frequencies on a graph. These peaks are as unique and reproducible as a fingerprint.
The number and position of these peaks in a spectrum can be predicted by quantum mechanics, but Fermi resonances result in unanticipated peaks due to a combination of two different vibrations, such as stretching and bending. First recognized in carbon dioxide and explained by Enrico Fermi in 1931, scientists agree that the Fermi peaks are the result of the mixing of the two vibrational modes, but they often label one of them as 'stretch' and the other as 'bend'. This labeling became a problem when PNNL researchers observed a 'flip' in the Raman spectrum of supercritical carbon dioxide.
Shift or Flip?
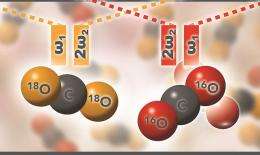
To follow reactions, researchers often use different versions of elements called isotopes. Normally, carbon dioxide contains carbon plus the isotope oxygen-16, the most common form of oxygen. By using a heavier isotope of oxygen with its own fingerprint, oxygen-18, PNNL researchers can track the fate of carbon dioxide when it reacts with minerals, particularly when there are other sources of oxygen present such as water.
In the Raman spectra of the lighter supercritical carbon dioxide, the pair of Fermi peaks included a weaker one at a lower frequency and a stronger one at higher frequency. When they replaced all of the oxygens with the heavier isotope, however, the peaks seemed to flip, with the stronger one appearing at a lower frequency instead.
At first, it was not clear how the two sets of Fermi peaks related to each other — whether the peaks were really a mirror image or if the stronger oxygen-16 peak somehow morphed into a weaker peak when heavy oxygen-18 was introduced. Typically, a heavier isotope will shift peaks to lower frequencies, although different modes are not necessarily affected by the same amount.
The researchers needed to unambiguously identify the peaks and to figure out how much bending and stretching modes contributed to each one. To do so, the team decided to simulate the carbon dioxide molecules with different oxygen isotopes on a computer and see if they could recreate the Raman spectra they saw in their experiments.
To the Computer
Using computing resources at EMSL, DOE's Environmental Molecular Sciences Laboratory at PNNL, Glezakou simulated carbon dioxide in supercritical conditions similar to those in the experiment. The molecules were "made" with either oxygen-16, oxygen-18.
They analyzed the motion of the molecules to produce computational spectra that echoed the real spectra. In this way, the team was able to determine the percent of bending and stretching modes expected in each peak.
The results showed that with oxygen-16, the stronger peak at the higher frequency is due mostly to the stretching mode, while the weaker peak at the lower frequency is due mostly to the bending mode.
Oxygen-18, however, told a different story. The results with heavy carbon dioxide showed unequivocally that the light- and heavy-oxygen peaks were not exactly mirror images of each other. Carbon dioxide is mostly a linear molecule, so the bending motion is much less affected than the stretch when the oxygen-16 is replaced by its heavier isotope. As a result, the composition of the peaks does not remain the same.
"The heavier oxygen doesn't just shift the peaks. It changes their identity," said Glezakou. "And the bigger effects are on the stretching, because the peak with the most stretching has the biggest frequency shift."
Windisch added that the experimental results matched the computational ones nicely, in spite of the difficulty. "Our colleague Paul Martin here at PNNL had to build equipment so we could do these experiments at the pressures we needed. Not easy," he said.
Having nailed down the vibrational pedigree of these carbon dioxide molecules, they plan to use these results to understand better other reactions between carbon dioxide and a variety of minerals.
More information: Reference: Charles F. Windisch Jr., et al., Raman spectrum of supercritical C18O2 and re evaluation of the Fermi resonance, Phys. Chem. Chem. Phys., November 14, 2011, DOI:10.1039/c1cp22349f
Journal information: Physical Chemistry Chemical Physics
Provided by Pacific Northwest National Laboratory