July 30, 2007 feature
Controlling nano color and shape with pH adjustments
Scientists have recently discovered that the shape, color, and optical properties of silver nanoparticles can be controlled using a method that is easy, inexpensive and takes just minutes. Simply by adjusting the pH value of the nanoparticles’ immersion solution, silver nanoprisms can be transformed into nanodiscs, which can also enhance the unique light scattering properties of the particles for possible applications.
The chemists from Northeast Normal University in China, Ying Chen, Chungang Wang, Zhanfang Ma (also with Capital Normal University) and Zhongmin Su, have demonstrated their method in a recent issue of Nanotechnology. The group showed how a more acidic solution decreases the wavelength of the silver nanoparticles’ absorption peaks, which can improve the so-called “enhancement mechanism” of Surface-enhanced Raman scattering (SERS). The scientists hope that this work will help lead to the fabrication of nanoparticle films for biosensing.
“This work will be of great significance in understanding the mechanism of morphology transitions of nanostructures with the changes of the surrounding environment,” Ma told PhysOrg.com. “Also, this work will be important in fabricating biosensing or chemosensing nanostructure films with different shapes using a simple routine method.”
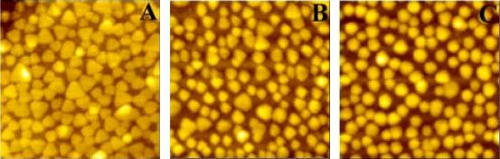
The scientists started with a batch of silver nanoparticles in the shape of prisms, with an average edge about 48 nm long, and appearing as a deep blue color under an atomic force microscope (AFM). When the scientists immersed the quartz substrate holding the nanoprisms into a solution with a pH of 5.0 for 5 minutes, the absorption peak shifted from about 800 to 500 nm, changing the color to deep purple. When immersed in a solution with a pH of 2.2, the absorption peak decreased to 432 nm, and turned yellow.
Besides the color change, the researchers were also surprised to find that the silver nanoprisms gradually changed into smaller nanodiscs as the pH decreased to below 6.0. The group attributes this shape change to the increased amount of hydrogen ions present in increasingly acidic solutions (pH is basically defined by the ratio of hydrogen ions, H, to hydroxide ions, OH, which is the breakdown of water).
Hydrogen ions can act as etchants, in essence carving away the sharp corners of the prisms, so much so that, eventually, the prisms become round discs. The lower the pH value, the stronger the etchant ability, and the more quickly the prisms become discs. The discs, with 35-nm diameters and 20-nm thicknesses, are also smaller than the prisms, resulting in a greater distance between the nanoparticles in a lower pH solution.
One of the many applications of controlling nano-sized color and shape, the scientists point out, is increasing the enhancement effects of SERS. SERS has been used in applications including materials analysis and amplification in telecommunications due to the ability to scatter light at different wavelengths than normal.
To enhance SERS, scientists can use nanoparticles, with their strong localized plasmon resonance, to amplify the localized electromagnetic field, which is called the electromagnetic effect. The ability to control the nanoparticles’ shapes allows researchers to achieve the particular excitation wavelength required to optimize this effect.
“Anistropic noble metal nanoparticulate substrates are better than spherical shaped nanoparticulate substrates for improving SERS enhancement,” Ma explained. “Our results will provide an easy route to fabricate the sensitive SERS substrate. This will be very useful not only for SERS biosensing applications—for example, protein and DNA detections—but also for chemosensing applications—for example, small chemical molecules detection.”
Citation: Chen, Ying, Wang, Chungang, Ma, Zhanfang, and Su, Zhongmin. “Controllable colours and shapes of silver nanostructures based on pH: application to surface-enhanced Raman scattering.” Nanotechnology 18 (2007) 325602 (5pp).
Copyright 2007 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.