August 27, 2007 feature
Carbon nanotubes' electronic properties optimized for future applications
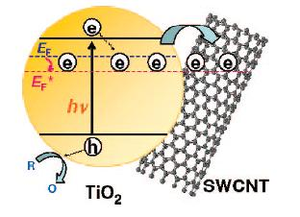
While researching the unique electrical properties of single-walled carbon nanotubes (SWCNTs), researchers have demonstrated the nanotubes’ ability to capture and store one electron per 32 carbon atoms in a SWCNT. The stored electrons can be readily discharged on demand with the addition of an electron-accepting dye, significantly increasing the photocurrent and photoconductivity of electrical systems.
University of Notre Dame scientists Anusorn Kongkanand and Prashant Kamat monitored the transfer of electrons from semiconductor particles to SWCNTs as the composite system strained to achieve charge equilibrium. The study, published in ACS Nano, will be useful for the design of nanotubes as a way to direct the flow charge and boost photoelectrochemical performance for applications including electronic devices and solar cells.
“Although the electron storage property of carbon nanotubes is well known, there is no convenient or simple way to make a quantitative estimate of storage capacity,” Kamat told PhysOrg.com. “Our study provides a quantitative measure of the number of electrons stored in carbon nanotubes and its ability to discharge them on demand. In addition, one can use the information to estimate the Fermi level of the semiconductor-carbon nanotube composite—an important parameter in evaluating the performance of SWCNT devices for electronic and photovoltaic applications.”
When excited by a UV laser, titanium dioxide nanoparticles undergo charge separation, where some of the semiconductor’s electrons get trapped—an estimated 3,770 electrons per 12-nm-long nanoparticle. Electrons trapped in the titanium dioxide displayed a blue coloration (a 650-nm absorption band).
But when the researchers introduced SWCNTs to the titanium dioxide particles, the blue color decreased. Because SWCNTs don’t have any detectable absorption in the visible range, this lack of color meant that some of the electrons trapped in the titanium dioxide were transferred to the SWCNTs.
“The transfer of electrons represents charge equilibration between the two semiconductor systems having different Fermi levels,” the scientists explained. “At a concentration of 100 mg/L SWCNT, we observe complete disappearance of the 650 nm absorption band, thus indicating complete transfer of electrons to SWCNT.”
Complete transfer consisted of 1 electron per 32 atoms of carbon atoms (building blocks of the SWCNTs), and occurred in just 10 nanoseconds. Such a high electron capacity turned the SWCNTs into supercapacitors, which can be useful in electronics applications.
“Boosting the electron storage in a tiny volume occupied by carbon nanotubes should be attractive for miniaturizing storage batteries,” Kamat said. “The electron transfer from semiconductor to the carbon nanotubes continues until the Fermi energies of the two match or equilibrate. Therefore, the estimate of the 32 electrons per carbon atom is limited by the energetics of the photoirradiated titanium dioxide system.
“By selecting another semiconductor particle with a more negative conduction band than that of TiO2 (in other words using a more energetically favorable semiconductor) or alternate charging methods (such as electrical or electrochemical charging), it should be possible to store more electrons,” Kamat explained. “The higher the energy level of the semiconductor, the greater the number of electrons transferred.”
Then to discharge the electrons, the researchers added thionine, a dye that acts as an electron acceptor. Electrons from the SWCNTs transferred to the thionine, which has a reduction potential that is more positive than the SWCNTs, causing charge equilibration to drive the electrons out of the nanotubes.
“The ability of SWCNTs to accept electrons and transfer them to a suitable electron acceptor highlights the mediating role of these nanotubes in a charge transfer process,” the researchers concluded. “This electron-charging and -discharging property of SWCNT will play an important role in improving the performance of light energy harvesting applications.”
Citation: Kongkanand, Anusorn, and Kamat, Prashant V. “Electron Storage in Single Wall Carbon Nanotubes. Fermi Level Equilibration in Semiconductor-SWCNT Suspensions.” ACS Nano, Vol. 1, No.1, 13-21, 2007.
Copyright 2007 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.





















