New research sheds light on shimmering superconductivity and the courtship of electrons
In their normal state, electrons repel each other because of their charge, but in the state of superconductivity, electrons pair up. John Schlueter, a chemist from the U.S. Department of Energy's Argonne National Laboratory, collaborated with a team of researchers from the University of Oxford to better understand how this unlikely courtship occurs.
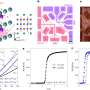
Their recent research appears in the October 4 issue of Nature and finds that a form of shimmering superconductivity exists at temperatures well above that at which ordinary superconductivity is destroyed. This electron courtship is characterized by a tension between the conflicting urges for electrons to pair up (which leads to superconductivity) and to repel each other (which leads to insulating behavior).
Superconductors conduct electricity with absolutely no resistance when cooled below a certain critical temperature, Tc. “Superconductivity already has important applications and many more uses are possible if critical temperatures are high enough,” Schlueter explained.
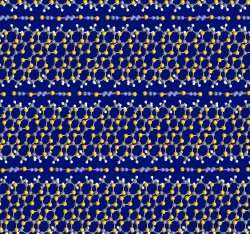
Much research over the past decade has focused on inorganic cuprate superconductors. Although molecular superconductors currently have maximum Tcs near 10 K (nearly an order of magnitude lower than the cuprates), they have many features that make them ideal for the study of the fundamental properties of superconductivity. In the organic materials, lower temperatures and magnetic fields are required to reach the boundaries between superconducting and normal states, thus making these experiments much easer to perform in a laboratory.
Although such shimmering superconductivity above the usual temperature barrier has previously been observed in cuprate materials, this is the first time it has been seen in an extremely clean and well controlled system that doesn't have to be chemically doped to produce superconductivity. This means that scientists can be sure that the effect is not associated with impurities. In fact, the team believes that such an effect should be found in all superconductors in which conflicting interactions are finely balanced. This is an important step forward in the quest to understand superconductivity in what are known as "highly correlated" materials: the superconductors of the future.
The Argonne group has long been recognized as an international leader in the discovery and crystallization of high quality crystals of molecular superconductors. “We use an electrocrystallization technique both as a discovery tool and a means to enable sophisticated measurements aimed at unraveling some of the outstanding mysteries of superconductivity,” Schlueter explained. This research was performed on a superconducting material discovered by the Argonne group in 1990, addresses a longstanding question relating to the pairing of electrons in superconducting materials. The study identifies similarities between the high and low temperature superconductors.
The discovery was made by Moon-Sun Nam in collaboration with Arzhang Ardavan and Stephen Blundell in Oxford University's Department of Physics, using crystals grown by John Schlueter. The team exploited a particularly sensitive probe of superconducting fluctuations called the "vortex-Nernst effect". This effect provides a way of detecting that superconducting vortices are present, even when zero electrical resistance (the characteristic of traditional superconductivity) is not exhibited.
Source: Argonne National Laboratory