FDA OKs novel surgical clotting solution
The U.S. Food and Drug Administration announced approval Thursday of the first clotting solution made using recombinant DNA techniques.
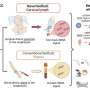
The FDA said the new solution, called Recothrom, is a topical solution designed to stop small blood vessels from bleeding following surgery. Recombinant DNA is the result of a genetic modification process that enables scientists to create new DNA strands with specific traits, such as the capacity to produce a specific protein.
The FDA said post-surgical bleeding from small blood vessels, such as capillaries, can cause significant blood loss. Physicians can apply Recothrom during surgery when standard surgical techniques for stopping blood loss are ineffective or impractical.
"With today's approval, surgeons can choose recombinant thrombin, thrombin derived from human plasma or thrombin derived from cattle plasma to help control surgical bleeding and oozing," said Dr. Jesse Goodman, director of the FDA's Center for Biologics Evaluation and Research.
Recothrom is made from Chinese hamster ovary cells that have been genetically modified to produce human thrombin. The cells are free from known infectious agents, the FDA said, noting Recothrom undergoes an additional process of viral inactivation.
Recothrom is manufactured by ZymoGenetics Inc. of Seattle.
Copyright 2008 by United Press International