Two microRNAs promote spread of tumor cells
The more scientists learn about microRNAs – short strands of RNA that can interfere with normal gene activity – the more obvious it becomes how closely they are associated with cancer. In a new study, scientists at The Wistar Institute and their colleagues have identified two microRNAs (miRNAs) that promote tumors’ deadly spread, or metastasis. One of the miRNAs may provide an early warning of metastatic breast cancer and the need for aggressive treatment.
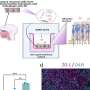
By blocking the translation of tumor suppressor genes, miRNAs have been shown to facilitate the development of many types of cancer. In a study that will be published February 1 in Nature Cell Biology and is available online, the researchers describe how two miRNAs transformed non-invasive human breast cancer cells into cells that rapidly metastasized in cell cultures and laboratory mice.
“Of the 450 miRNAs we tested, we found two, miR-373 and miR-520c, that induced cell migration in MCF-7 cells – a line of human breast cancer cells that normally does not metastasize,” says Qihong Huang, M.D., Ph.D., an assistant professor in Wistar’s Molecular and Cellular Oncogenesis Program and lead author and co-corresponding author on the study.
In 2006, miR-373 was identified as a possible oncogene – a modified gene that causes cancer – in testicular cancer. According to Huang, miR-520c is a new miRNA whose function has not been known until now.
“Our most surprising finding is that miR-373 and isoforms of miR-520 are part of the same family,” Huang says. “Their seed sequences, or first eight nucleotides, are all very similar. It suggests this family of miRNAs could target similar genes and have important biological and pathological functions in cancer development and metastasis.”
Another intriguing characteristic of these two miRNAs is that they are not found in normal adult cells – only in tumor cells. “They are not in normal testis, but are expressed in testicular cancer,” Huang says. “We see them in breast cancer cells, especially metastatic cells, but not in normal breast cells.”
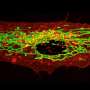
After they confirmed the metastasis-inducing properties of the miRNAs, the research team began searching for their target genes in MCF-7 cells. Several experiments limited the search to a gene called CD44, which contains genetic instructions for a common cell surface receptor molecule. Found in most cell types, CD44 affects cell-cell interactions and interactions between cells and their microenvironments. It also has been shown to inhibit tumor metastasis.
When CD44 was downregulated, non-metastatic MCF-7 cells became metastatic. When the scientists injected MCF-7 cells without CD44 into immunodeficient mice, the mice developed bone and lung tumors, while mice receiving MCF-7 cells with CD44 did not.
“We found that miR-373 and miR-520c interfered with the expression of CD44 in MCF-7 cells,” Huang says. “We think there are additional targets involved, but our results suggest that these miRNAs promote cell metastasis at least in part by limiting the expression of CD44.”
In the final stages of the study, Huang and his colleagues analyzed 11 pairs of primary and metastatic breast cancer tissue samples from cancer patients. The scientists found that metastatic tumors removed from lymph nodes contained more miR-373 than the primary breast tumor from the same patient.
An additional study of 72 human primary breast tumors found higher mean expression of miR-373, and lower mean expression of CD44, in primary tumors from patients whose cancer had spread to their lymph nodes compared to patients whose tumors had not spread.
According to Huang, these results suggest miR-373 has the potential to become an important early biomarker for metastatic breast cancer. “As far as we know, miR-373 is not expressed in normal tissue,” Huang says. “So if we detect miR-373 in lymph nodes when a patient’s breast tumor is removed, it would indicate that cancer cells have spread and the patient will need more aggressive therapy.”
Huang plans additional studies to explore the use of miR-373 in the diagnosis of metastatic breast cancer. Also, because he has found the inhibition of miR-373 to reduce tumor migration and invasion, he plans to investigate its potential to treat breast cancer and other cancers.
Source: The Wistar Institute