May 8, 2008 feature
Researchers Observe Hydrogen-Bond Exchange
Hydrogen bonds are quite small, on the level of a few angstroms. They can also be passed between two different molecules very quickly, at speeds of tens of times per second. But in spite of these properties, researchers have recently observed hydrogen-bond exchange taking place in real-time.
In their study published in Physical Review Letters, a team of chemists from Kyoto University and Osaka University, both in Japan, describe how they used a scanning tunneling microscope to directly observe a hydrogen-bond exchange taking place within a single water dimer (two molecules of H2O bound together). The observation provides evidence to support the model in which quantum tunneling and molecular vibrations play important roles in the hydrogen-bond exchange process.
“The dynamics of hydrogen-bond exchange is visualized at single-molecule level in this work,” Hiroshi Okuyama of Kyoto University told PhysOrg.com. “Previous studies used spectral splittings as evidence for the exchange reaction. This [direct observation] enabled us to clearly show that the exchange process involves quantum tunneling and also can be promoted by correlated vibrational excitation. This work is, I believe, of fundamental importance.”
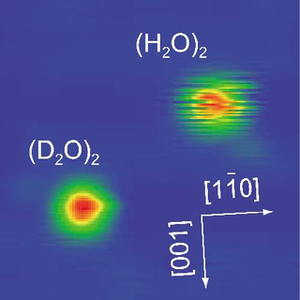
Because water dimers are the simplest system containing a hydrogen bond, researchers often use them to investigate general properties of hydrogen-bonding systems. In the current experiment, the researchers prepared water dimers from water monomers (single H2O molecules) on a copper surface at a cold 6 degrees K. The water dimers bonded to the copper surface via the oxygen atom of its “hydrogen-bond donor” molecule, along with its “hydrogen-bond acceptor” molecule weakly interacting with the adjacent copper atom. The researchers also substituted some of the H20 dimers with heavy water deuterium dimers, (D20)2, for comparison.
While the D20 dimers appeared stationary under the microscope, the H20 dimers fluctuated continuously. As the scientists explained, these fluctuations represent the rapid exchange of hydrogen bonds between the two H20 molecules as they interchange their roles of donor and acceptor molecules. By rearranging the hydrogen bond that holds them together, the molecules switched back and forth between these two roles at a rate of about 60 times per second. The reason that the D20 dimers didn’t appear to fluctuate under the microscope was because their hydrogen-bond exchange rate was much slower, at just 1 time per second.
This rate difference, which the researchers call an isotope effect, means that the interchange must overcome a large energy barrier, one that is too large for mere thermal processes. It implies that the interchange proceeds instead via quantum tunneling. As the researchers explain, the oxygen atoms on the molecules must be displaced in such a way as to bring the acceptor and donor molecule to nearly the exact same height – close enough for tunneling to occur. As the molecules are vibrationally excited in such a way that the displacement occurs, the interchange rate is drastically increased.
By observing the hydrogen-bond exchange in the water dimers, scientists can better understand the role it plays in many applications. One of these areas, Okuyama explained, is the catalytic reactions that take place at electrode surfaces, such as in fuel cells.
“The fuel cells mounted in future (and present) cars will reduce their exhaust gasses,” he said. “The basic idea of the fuel cell is an electrochemical reaction at the electrode surfaces, where complex reactions proceed with the reactants and products including solvent water. At the microscopic level, water molecules adsorb at the electrode surface, and dynamically form, break and exchange their hydrogen bonds with each other, probably promoting the electrode reaction.”
More information: Kumagai, T.; Kaizu, M.; Hatta, S.; Okuyama, H.; Aruga, T. “Direct Observation of Hydrogen-Bond Exchange within a Single Water Dimer.” Physical Review Letters 100, 166101 (2008).
Copyright 2008 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.





















