Fruit fly protein acts as decoy to capture tumor growth factors
Researchers at the University of Pennsylvania School of Medicine have shown how Argos, a fruit fly protein, acts as a 'decoy' receptor, binding growth factors that promote the progression of cancer. Knowing how Argos neutralizes tumor growth may lead to new drug designs for inhibiting cancer. The study appeared online in Nature in advance of print publication.
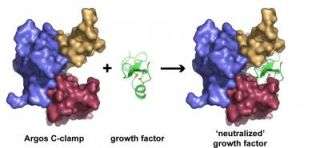
Many types of tumors grow because of over-expression of a protein known as the epidermal growth factor receptor (EGFR) or a peptide hormone called epidermal growth factor (EGF) that binds and activates EGFR. Argos mimics EGFR by binding to EGF. But, unlike EGFR, Argos does not signal cells to grow.
In theory, surmise the researchers, a drug designed to resemble Argos could bind cancer growth factors and prevent them from signaling cancer cell growth. The investigators previously found that Argos works this way in the fruit fly, binding and neutralizing the fly version of EGF called Spitz. Inhibition of Spitz in this way is crucial for proper development of the fly eye.
"There are several ¡¥designer' cancer drugs that fight tumors driven by EGFR-like receptors, such as Herceptin, Erbitux and Tarceva," says lead author Mark A. Lemmon, PhD, Professor of Biochemistry and Biophysics. "Whereas these drugs all attack the receptor itself, an Argos-like drug would instead neutralize the cancer growth factor by mimicking a silent receptor. This is a change in paradigm for tumor-growth inhibition in this class of cancers."
Approaches using molecules that neutralize growth factors have proven themselves in other cases. The Avastin antibody works well to block the molecule that activates the vascular endothelial growth factor receptor and several drugs can block tumor necrosis factor-Ą in arthritis, including Remicade, Humira and Enbrel. An Argos-like drug would work the same way in EGFR signaling, suggests Lemmon.
In the current study, Lemmon and colleagues have worked out the details of the three-dimensional structure of Argos when it binds to Spitz. "We were surprised to find that Argos has three very similar domains that capture Spitz by surrounding it like a C-clamp," explains Lemmon. Although Argos binding to Spitz mimics the characteristic binding of EGFR to EGF, Argos and EGFR do not share the same amino acid sequence or structural similarities.
The structure of Argos was studied by X-ray crystallography, a technique that shows where each atom of the protein is located. Computer analysis is then used to put together all the data into a three-dimensional projection of the growth factor and its binding molecule.
The next step is to identify a human protein that is similar in structure to Argos. Intriguingly, Argos shares structural similarities with the human receptors for transforming growth factor ƒÒ and urokinase plasminogen activator. "There are quite a few other human proteins with similar predicted structures whose function is not yet known," says Lemmon. "We think that one of these might be a functional analogue of Argos, and we could exploit that as a drug."
Source: University of Pennsylvania