New insights into thalidomide-birth defect episode
Scientists in Germany have discovered why the medication thalidomide appeared safe in animal tests before going on the market 50 years ago, only to cause perhaps the most extensive outbreak of drug-induced birth defects in medical history. Their study is scheduled for the December 1 edition of ACS' Molecular Pharmaceutics.
Jurgen Knobloch, Ulrich Ruther and colleagues note that more than 10,000 children were born with severe birth defects after drug regulators in Europe approved the medication for treating nausea and vomiting in pregnant women. The drug, never approved for that use in the United States, is available for certain conditions, including multiple myeloma and leprosy. The birth defects outbreak puzzled scientists because pre-marketing tests in lab mice and rats showed no sign of a birth defect risk.
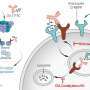
The researchers point out that those animals proved to be resistant to thalidomide's adverse effects, and in the new study they describe discovery of the biochemical basis for that resistance. It involves a key difference between human embryonic cells and those of mice. They found in mice cells advanced antioxidant defenses compared to those in humans and other thalidomide-susceptible species.
Therefore, thalidomide is not able to induce the generation of large quantities of damaging free radicals called superoxides in mouse embryonic cells as it does in human embryonic cells (where subsequent cell death is believed to be responsible for birth defects.)
Article: "Thalidomide Resistance Is Based on the Capacity of the Glutathione-Dependent Antioxidant Defense", dx.doi.org/10.1021/mp8001232
Source: ACS