Structure of a virulent pathogen revealed
(PhysOrg.com) -- Like high-profile politicians, pathogenic bacteria dispatch advance teams to make way for their arrival. But these bacterial agents don’t just secure a safe passage, as a Secret Service detail might do. Rather they are teams of molecules that bacteria inject into cells they want to colonize, sent to hijack their hosts’ biochemistry to serve their master’s microbial needs. These molecules — called virulence factors — co-opt essential cell functions including the reproduction cycle and cell structure assembly, suppressing the cells’ defenses against bacterial invasion and causing disease.
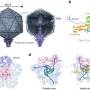
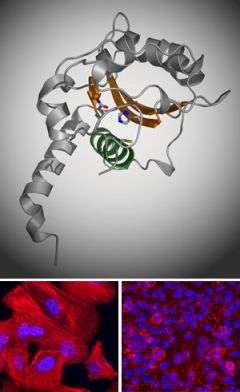
Now researchers at The Rockefeller University have revealed the crystal structure of one virulence factor common to the meaner strains of Escherichia coli that are the leading causes of dangerous bouts of diarrhea in developing countries. The structure, a kind of molecular image that shows the position and identity of every atom in the so-called cycle inhibiting factor (Cif), offers clues as to how this particular bacterial weapon works and, potentially, how to defend against it or even use it to attack cancer.
“Cif shuts down cell cycle progression in a way we don’t yet understand. If we can figure this out, we may be able to find ways to inhibit the cell cycle in certain tissues that we don’t want to grow, like tumors,” says C. Erec Stebbins, associate professor and head of the Laboratory of Structural Microbiology at Rockefeller. The research of Stebbins and his colleagues, to be published in the December 12 edition of the Journal of Molecular Biology, is a significant advance toward understanding exactly how Cif manipulates the cells it invades.
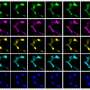
Using the century-old, labor-intensive technique of x-ray crystallography to identify the structure of the protein, researchers showed Cif is part of a prominent “superfamily” of enzymes including cysteine proteases and acetyltransferases. Like these enzymes, Cif contains three amino acid residues — a catalytic triad — that are essential to its virulence. Stebbins and colleagues used point mutations to remove each of these residues and then tested the mutants for whether they could arrest the cell cycle. When test cells are exposed to bacteria with regular Cif, they puff up, form tell-tale stress fibers and are essentially frozen at the stage of cell reproduction just before mitosis. None of the mutants had this effect on the host cells, however, showing that the residues were critical to Cif’s work.
Further work will aim to elucidate what part of the cell Cif targets as well as its exact molecular mechanism of action. “Once we get the target for Cif’s enzymatic activity, we will determine how it arrests the cell cycle, and that could teach us a way to manipulate the cell cycle ourselves,” Stebbins says.
Citation: Journal of Molecular Biology 384(2): 465–477 (December 12, 2008)
dx.doi.org/10.1016/j.jmb.2008.09.051
Provided by Rockefeller University