Research shows cell's inactive state is critical for effectiveness of cancer treatment
A new study sheds light on a little understood biological process called quiescence, which enables blood-forming stem cells to exist in a dormant or inactive state in which they are not growing or dividing. According to the study's findings, researchers identified the genetic pathway used to maintain a cell's quiescence, a state that allows bone marrow cells to escape the lethal effects of standard cancer treatments.
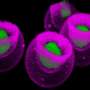
Researchers at Memorial Sloan-Kettering Cancer Center (MSKCC) found elevated levels of the tumor suppressor protein p53 in hematopoietic stem cells (HSCs) - immature cells in the bone marrow that have the capacity to differentiate into all types of mature blood cells. They showed that when chemotherapy or radiation is delivered to a cell that lacks both p53 and a gene called MEF, the cell not only becomes less quiescent, but also becomes more susceptible to being killed. These findings are published in the January 9, 2009, issue of Cell Stem Cell.
"This is the first time that anyone has established that p53 has a role in defining a cell's state of quiescence. Furthermore, it is surprising that some cells that lose p53 can actually be killed more readily than those that have p53 intact," said the study's senior author, Stephen Nimer, MD, Chief of the Hematology Service and Member of the Molecular Pharmacology and Chemistry Program at MSKCC. "Our findings have important implications for developing therapeutic strategies that could eliminate quiescent cancer stem cells."
The study builds on previous research in which Dr. Nimer and colleagues first identified the MEF gene and showed its ability to control the state of quiescence of HSCs as well as its critical role in determining the sensitivity of normal bone marrow cells to chemotherapy and radiation. They have now identified p53 as the pathway that MEF utilizes to maintain this enhanced quiescence.
It is known that when a cell experiences DNA damage as a result of cancer treatment, p53 plays a critical role in guarding the genomic integrity of the cell by either triggering it to die or by causing cells to stop growing so they can repair their DNA successfully. However, p53 has additional functions during the process of blood cell formation in the body - a process called hematopoiesis.
In the current study, investigators set out to determine whether the increased amount of p53 and enhanced expression of p53 target genes might contribute to the quiescence of cells and their ability to resist chemotherapy. They examined the function of p53 during hematopoiesis and found an important interdependency between p53 and its target gene, MEF, on HSC quiescence.
"Our findings suggest that by targeting those specific genes that control quiescence in cancer cells, we may enhance the anticancer effects of chemotherapy and radiotherapy, thereby promoting their effectiveness," said Dr. Nimer.
In addition, researchers identified two new targets of the p53 protein - Necdin and Gfi-1 - tumor growth suppressor genes that also regulate quiescence. Researchers lowered the expression of Necdin and Gfi-1 in hematopoietic stem cells lacking MEF and found a significant reduction in the quiescence of those cells. The results suggest that these p53 target genes are functionally responsible for the enhanced quiescence of HSCs in which MEF has been eliminated.
Source: Memorial Sloan-Kettering Cancer Center