February 16, 2009 feature
Easing Atmospheric CO2 Levels Using Nanotubes and Sunlight

(PhysOrg.com) -- Researchers at The Pennsylvania State University have determined a way to use arrays of nanotubes in a solar-based process to convert carbon dioxide and water into methane and other hydrocarbon fuels. Their method may provide a new way to reduce carbon-dioxide levels in the atmosphere—rising due to our planet's heavy use of fossil fuels—as well as produce alternative fuels.
The rate of carbon dioxide (CO2) conversion using this method is 20 times higher than that of previously published research. The work is described in the January 27, 2009, online edition of Nano Letters.
"Every 12 days the world consumes about one billion barrels of oil, which represents the release of almost 1 trillion pounds of carbon dioxide into the atmosphere," said the study's lead researcher, Craig Grimes, to PhysOrg.com. "One way of dealing with this problem is by recycling the CO2 into a high-energy-content fuel, but this makes sense only if a renewable energy source, like solar energy, can be used in the process."
This type of solar-based conversion process only works if a photocatalyst—a material that reacts with light—is used to convert the CO2 into hydrocarbons. A photocatalyst that utilizes the most solar energy possible is the best option.
One popular photocatalyst candidate for the job has been titanium dioxide, also called titania, because it can powerfully react with oxygen. But so far, researchers haven't been able to make titania perform adequately despite experimenting with a variety of forms, such as nanoparticles, pellets, and multi-layer films.
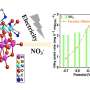
Grimes and his colleagues used arrays of titania nanotubes. They created the nanotubes using a technique that incorporates nitrogen into the nanotubes' structures, which the researchers initially thought would help increase the conversion rate (this turned out to be true only in a very limited capacity).
The process also yields a high total surface area compared to other forms of the material, a property that aids in the conversion. To further boost the process, the group scattered an ultra-thin layer of platinum and/or copper "cocatalyst" nanoparticles on the surface of the array.
The nanotubes were as long as 140 micrometers (millionths of a meter) in length and a diameter of about 115 nanometers (billionths of a meter). The total size of each array sample was about 2 centimeters square and the group created several samples.
The researchers made two reaction chambers, each with a window at the top to let in sunlight. They loaded one sample into each chamber and evacuated the air out, producing a vacuum, and sealed them. Next they pumped CO2 through a tank of water and into the chambers, flushing it through via intake and outtake valves for 10 minutes.
This all took place outdoors on sunny or mostly-sunny days on the Penn State campus. The samples were left outside for 2.5 hours, up to a maxiumum of 3.5 hours, between about 12:30 and 4:00 p.m.
Analysis of the chambers' interiors showed that the predominant product of the conversion was methane, with some ethane, propane, butane, pentane, and hexane, along with other materials in very small concentrations. The conversion rates were high, although comparing the results with other published results was rather difficult, according to the group.
"Most of the previous results were achieved using nanoparticles illuminated by ultraviolet light, so we were not exactly comparing apples to apples," said Grimes. "But going by the weight of the material, we could figure out that the rate of hydrocarbon production we achieved is at least 20 times higher than those previous studies."
Grimes and his group attribute their success, in large part, to the cocatalyst particles they used. They think that a homogeneous distribution of both types could further increase the conversion rate.
More information: Nano Lett., 2009, 9 (2), pp 731-737 DOI: 10.1021/nl803258p
Copyright 2009 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.