Pinpointing catalytic reactions on carbon nanotubes
(PhysOrg.com) -- Among their many other interesting properties, carbon nanotubes have been found to act as catalysts for some important chemical reactions, including some that could be used to make cleaner fuels. But many unanswered questions remain about how this process works.
Cornell researchers have answered an important one by pinpointing unique sites where the reactions take place on single-walled nanotubes. But directly observing these sites has been challenging, but now, the researchers have shown that the reactions do not occur all along the tubes, but at the ends of the tubes or at defects along the tubes.
The research by Peng Chen, Cornell assistant professor of chemistry and chemical biology, and his research group was reported April 14 in the online edition of the journal Nano Letters and will appear in a forthcoming print edition.
Carbon nanotubes are microscopic cylinders with walls made of carbon atoms arranged in connected hexagons, somewhat like a rolled up tube of chicken wire. A typical nanotube is a few nanometers (nm) in diameter and several microns long. (A nanometer is one-billionth of a meter, about as long as three atoms in a row. A micron is one-millionth of a meter, or about three times the diameter of a human hair.) Chen's observations have located catalytic reaction sites to within about 20 nm.
Nanotubes act as catalysts when an electric current is passed through them. This enables them to donate electrons to molecules that come in contact with the reaction sites. The reaction is similar to what happens in fuel cells, Chen said, so further research may help in making better fuel cells.
Other researchers at Cornell and elsewhere have shown that carbon nanotubes can be made into transistors. Thus, one long-range goal, Chen added, is to make them into photoelectrochemical cells that would use sunlight directly to make hydrogen.
"We want to use photons to make electrons, then use the electrons in a water-splitting reaction to make hydrogen," he explained, noting that this would help deal with the storage and transportation problems that have hindered the use of solar energy.
Fortuitously, another reaction that carbon nanotubes can catalyze changes a chemical called resazurin into another, resorufin, that is fluorescent. Under a microscope, tiny flashes of light reveal when and where the fluorescent molecules have been created.
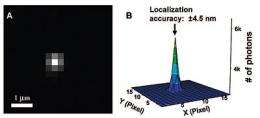
Chen's research group trapped an array of nanotubes between transparent conductors in a solution of resazurin and made a "movie" with an exposure every 100 milliseconds over tens of minutes after applying a voltage to start the catalytic reaction. A scattering of bright dots in each frame shows that the reactions are not happening all along the tubes.
Each dot is made up of thousands of photons, and because a light microscope typically cannot resolve features smaller than the wavelength of the light used -- in this case about 400 nm -- they appear scattered. So the researchers used an ingenious mathematical trick, plotting the rise and fall of brightness across each fuzzy dot to pinpoint the center. Think of finding the center aiming point of a shotgun by measuring the distribution of the pellets. Finally they superimposed the centers from all the frames of the movie and repeated the process to refine the locations to within 20 nanometers or less.
"The question now is what are the chemical natures of the reaction sites," Chen said. "Can we see how the electron transfer works?" Now that the sites can be located, he said, it will be possible to use high-resolution scanning tunneling microscopy to observe their atomic structure and relate their structure to electron transfer properties.
Provided by Cornell University (news : web)