How Solid Is Concrete's Carbon Footprint?
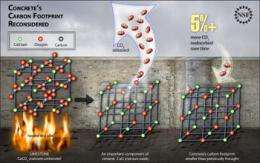
(PhysOrg.com) -- Many scientists currently think at least 5 percent of humanity's carbon footprint comes from the concrete industry, both from energy use and the carbon dioxide (CO2) byproduct from the production of cement, one of concrete's principal components.
Yet several studies have shown that small quantities of CO2 later reabsorb into concrete, even decades after it is emplaced, when elements of the material combine with CO2 to form calcite.
A study appearing in the June 2009 Journal of Environmental Engineering suggests that the re-absorption may extend to products beyond calcite, increasing the total CO2 removed from the atmosphere and lowering concrete's overall carbon footprint.
While preliminary, the research by civil and environmental engineering professor Liv Haselbach of Washington State University re-emphasizes findings first observed nearly half a century ago--that carbon-based chemical compounds may form in concrete in addition to the mineral calcite-now in the light of current efforts to stem global warming.
"Even though these chemical species may equate to only five percent of the CO2 byproduct from cement production, when summed globally they become significant," said Haselbach. "Concrete is the most-used building material in the world."
Researchers have known for decades that concrete absorbs CO2 to form calcite (calcium carbonate, CaCO3) during its lifetime, and even longer if the concrete is recycled into new construction--and because concrete is somewhat permeable, the effect extends beyond exposed surfaces.
While such changes can be a structural concern for concrete containing rebar, where the change in acidity can damage the metal over many decades, the CaCO3 is actually denser than some of the materials it replaces and can add strength.
Haselbach's careful analysis of concrete samples appears to show that other compounds, in addition to calcite, may be forming. Although the compounds remain unidentified, she is optimistic about their potential.
"Understanding the complex chemistry of carbon dioxide absorption in concrete may help us develop processes to accelerate the process in such materials as recycled concrete or pavement. Perhaps this could help us achieve a nearly net-zero carbon footprint, for the chemical reactions at least, over the lifecycle of such products."
That is the thrust of Haselbach's current NSF-funded work, where she is now looking at evaluating the lifecycle carbon footprint of many traditional and novel concrete applications, and looking for ways to improve them.
"This work is part of the portfolio of studies that NSF is funding in this vital area," added Bruce Hamilton, director of NSF's environmental sustainability program and a supporter of Haselbach's work. "Research relating to climate change is a priority."
Source: National Science Foundation (news : web)

















