Ice Gets Bent Out of Shape
For the first time, scientists have built completely flat, two-layer ice. While theoreticians have predicted that such ices are formed by squeezing water molecules between two surfaces, scientists at Pacific Northwest National Laboratory and Ruhr-Universitat Bochum are the first to create it. All it took was collaboration, creativity, and the absence of pressure.
Creating this type of ice shows the structure of water on a water-fearing, or hydrophobic, surface. The structure of water at these surfaces is vital to understanding protein folding, and the assembly of cellular membranes and intracellular compartments.
Close collaboration between theoreticians and experimentalists along with well-equipped laboratories and a supercomputer allowed the team to build a layer of ice two molecules thick and then to determine how the atoms and electrons in that ice arranged themselves.
The experimentalists began with a thin sheet of carbon, known as graphene on top of a layer of platinum. Then, they introduced a small amount of water onto the surface in ultrahigh vacuum (that is, no pressure) and very low temperatures. While ice traditionally forms at 273 Kelvin, for this experiment, the temperature was dropped to 125 K, about the temperature of an evening on the moon.
Next, the team used low-energy diffraction equipment that sends waves of slow-moving electrons at the surface. How those electrons bounce off the surface tells researchers a lot about the structure of the material.
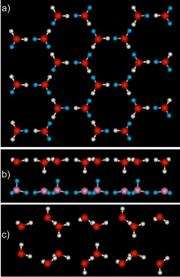
The researchers found a layer of smooth ice had grown on the graphene, not the usual puckered layers of ice seen on water friendly or hydrophilic surfaces. In the new ice, the angles between the atoms in the water molecules were stretched or compressed compared to normal ice. "This makes for stressed ice," said Dr. Greg Kimmel, the team lead.
So, the team subjected the ice to infrared spectroscopy, giving them information on how the water molecules were vibrating in the ice film. These results confirmed that the water molecules were in unusual configurations.
With these results, the theoreticians did complex calculations that determined the position of each of the atoms in the ice layer. The calculations were done with the software NW-ICE on a supercomputer at the Department of Energy's EMSL, a national scientific user facility.
The theoreticians found that in each layer of the ice, the water molecules formed slightly larger rings than normal. These six-sided rings stacked on top of each other, when looking down through the layers. They also found that each water molecule formed four hydrogen bonds—three with other molecules in the same layer, and one with water in the other layer. They also found that there are two types of water molecules: One type is parallel to the surfaces of the ice and its H-O-H angle is larger than normal. The other type connects the two layers and its H-O-H angle is smaller than normal.
The team, again integrating experiments and theory, are studying the temperature range over which the two-layer ice is stable and what happens when more water is added on top of the two-layer ice.
The research was done by Greg A. Kimmel, Jesper Matthiesen, Christopher J. Mundy, Nikolay G. Petrik, R. Scott Smith, Zdenek Dohnlek, and Bruce D. Kay at Pacific Northwest National Laboratory and Marcel Baer at Lehrstuhl fr Theoretische Chemie, Ruhr-Universitat Bochum in Germany.
Experiments and calculations were done in DOE's EMSL, a national scientific user facility at PNNL.
More information: Kimmel GA, J Matthiesen, M Baer, CJ Mundy, NG Petrick, RS Smith, Z Dohnalek, and BD Kay. 2009. "No Confinement Needed: Observation of a Metastable Hydrophobic Wetting Two-Layer Ice on Graphene." Journal of the American Chemical Society 131(35):12838. pubs.acs.org/journal/jacsat
Provided by PNNL


















