Study shows neural stem cells in mice affected by gene associated with longevity
(PhysOrg.com) -- A gene associated with longevity in roundworms and humans has been shown to affect the function of stem cells that generate new neurons in the adult brain, according to researchers at the Stanford University School of Medicine. The study in mice suggests that the gene may play an important role in maintaining cognitive function during aging.
"It's intriguing to think that genes that regulate life span in invertebrates may have evolved to control stem cell pools in mammals," said Anne Brunet, PhD, assistant professor of genetics. She is the senior author of the research, which will be published Nov. 6 in Cell Stem Cell.
Unlike your skin or your intestine, your adult brain doesn't make a lot of new cells. But those it does are critical to learning, memory and spatial awareness. To meet these demands, your brain maintains two small caches of neural stem cells, which can both self-renew and give rise to neurons and other cells known as oligodendrocytes and astrocytes. Properly balancing these functions allows you to generate new nerve cells as needed while also maintaining a robust neural stem cell pool.
As mice and other organisms age, the pool of neural stem cells in the brain shrinks and fewer new neurons are generated. These natural changes correlate with the gradual loss of cognitive ability and sensory functions that occur as we approach the end of our lives. However, the life span of some laboratory animals can be artificially extended by mutating genes involved in metabolism, and some humans outlive their life expectancy (about 70 years for someone born in 1960) by decades. Brunet and her colleagues wanted to know why.
The researchers studied a family of transcription factors called FoxO known to be involved in proliferation, differentiation and programmed cell death. FoxO genes are required for the extreme longevity seen in some strains of laboratory roundworms, and a single mutation in the FoxO3 gene has recently been associated with long life in Japanese, German, American and Italian populations.
"We wanted to know if FoxO3 could be involved in regulating the pool of neural stem cells," said Brunet. To do so, the researchers examined laboratory mice in which the FoxO3 gene was knocked out. While mice can live without FoxO3, such mice usually die from cancer between 12 and 18 months after birth. The normal life span of a laboratory mouse is about 30 months.
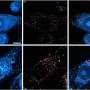
Brunet and her colleagues used mice of three different ages, both with and without the gene: 1-day-old (newborns), 3-month-old (young adult) and 1-year-old (middle age). They found that, overall, adult and middle-aged mice without FoxO3 had fewer neural stem cells than did age-matched mice with this regulatory protein. There were no significant differences between the newborn mice with and without FoxO3, suggesting that FoxO3 loss only affects adults.
The researchers also discovered that the few stem cells found in the adult mice without FoxO3 more rapidly churned out neural cell precursors — those cells destined to become new neurons — than did the mice with normal FoxO3 levels. In fact, the brains of the mice that lacked FoxO3 were heavier than the control group, perhaps because they were burning through their pool of neural stem cells by making too many new nerve cells.
When the researchers looked at the neural stem cell in a laboratory dish, they found that those from young and middle-aged adult mice lacking FoxO3 — but not those from newborn mice — seemed to be compromised in their ability to self-renew and to generate the three types of nerve cells. Further investigation bolstered their findings when they discovered that the FoxO3 protein regulates the expression of genes involved in quiescence and differentiation in cells.
The researchers concluded that FoxO3 may be needed for the stem cells to re-enter a waiting state called quiescence that normally occurs after dividing. Cells that are unable to enter quiescence are less able to self-renew and may lose their ability to become any of the three nerve cell types.
Together, the research results suggest that FoxO3 is important to regulate the pool of neural stem cells in the adult brain.
Although the researchers studied mice of varying ages, from birth to about one year, Brunet stressed that their current study does not address changes that might occur in FoxO3 levels or activity over time — a technically difficult endeavor they are now pursuing.
"We suspect that indeed there will be some changes," said Brunet. "But they will be relatively subtle. We know that the level of FoxO3 doesn't vary drastically, but it's possible the protein becomes less active over a mouse's life span. Or perhaps it simply becomes overwhelmed by the accumulated molecular changes of aging."
Brunet and her colleagues, along with collaborators at the University of Arkansas, are working on creating a mouse in which FoxO3 levels are artificially elevated. If their theory about the function of the protein in the brain is correct, it's possible that the neural stem cell pools of these mice will be protected from the ravages of time.
"We're very interested in understanding how everything unravels during the aging process," said Brunet.
Source: Stanford University Medical Center (news : web)