Fighting climate change by turning CO2 to stone

(PhysOrg.com) -- While politicians debate the best ways to cut global carbon dioxide emissions, researchers at Idaho National Laboratory's Center for Advanced Energy Studies are charging ahead on a strategy to defuse the CO2 the world already produces. They want to inject the greenhouse gas deep underground, where it would react with rocks and remain, entombed, for thousands of years.
CAES scientists have been studying this novel approach — called mineral sequestration — for years. They have characterized promising injection sites and run many computer simulations to understand how the process works. But they will soon ramp up their efforts dramatically, thanks to collaborations with international research groups, newly installed lab equipment and a recently awarded $750,000 grant. The CAES team will play a key role in determining if mineral sequestration is a viable strategy for mitigating the impact of climate change — or just a pipe dream.
"The next year ought to be pretty exciting for us," says geochemist Travis McLing, INL's technical lead for carbon capture and sequestration. "The rubber should really hit the road."
What to do with all that CO2?
Over the past 150 years, atmospheric levels of heat-trapping CO2 have increased by 35 percent, chiefly as a result of intense fossil-fuel use. During this same period, average global temperatures have risen by 0.6 to 0.9 degrees Celsius. Many climate scientists have long argued that the world risks a climate catastrophe if it continues to pump out so much CO2, and politicians have begun to agree that something needs to be done.
The ultimate goal is to switch the global energy economy over to cleaner, greener sources. But this fix is years down the road and fraught with economic and technological hurdles. In the short term, burning fossil fuels is far cheaper than developing renewable energy resources (such as solar and wind) or building more nuclear power plants. Petroleum, coal and natural gas still generate 84 percent of the energy consumed in the United States, according to the Department of Energy's Energy Information Administration. And the EIA estimates that global CO2 emissions will grow by 1.4 percent every year through 2030.
But not all CO2 we produce has to end up in the atmosphere. Carbon dioxide generated by "point sources" such as power plants — as opposed to more dispersed emitters like cars and planes — can be captured before it leaves the smokestack. This CO2 can then be injected hundreds or thousands of meters underground, sealed safely away for many years.
Some scientists believe this strategy, termed carbon capture and sequestration (CCS), could help the world buy some time while it figures out a long-term energy solution. And CCS has moved beyond the realm of the purely theoretical. StatoilHydro, a Norway-based energy company, has been injecting 1 million tons of CO2 underground annually since 1996.
A more permanent solution
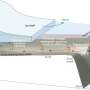
StatoilHydro injects CO2 into porous sandstone 800 meters (about 2,600 feet) beneath the North Sea floor. The overlying rock is gas-tight, the company says. But some researchers, such as McLing and fellow INL scientist Rob Podgorney, would prefer to remove any possibility that CO2 could escape back into the atmosphere. That's why they're working on the mineral sequestration side of CCS. Their goals are both fundamental and ambitious.
"We want to know what exactly is going on underground when you inject CO2," Podgorney says.
The basic theory is well understood. Certain types of rock, such as basalt, are rich in metallic ions like calcium, magnesium and iron. When CO2 is injected deep into basalt formations, it dissolves in water and reacts with these ions to produce carbonate minerals (such as calcium carbonate). CO2 is thus locked into solid, stable rock.
The potential of this process is huge — basalt makes up about 65 percent of the Earth's crust. According to a recent paper in the journal Energy Procedia, the Juan de Fuca tectonic plate off the United States' northwest coast could suck up 700 billion tons of CO2 by itself — far more than the 33 billion tons produced by humans every year.
McLing, Podgorney and their colleagues — researchers at the University of Idaho and Idaho State University, all working together under the CAES umbrella — are among a few groups in the world investigating mineral sequestration in depth. The CAES scientists have drawn up models predicting how the approach would work, filling in key details by studying the basalt fields underlying Idaho's Snake River Plain. Their simulations are encouraging, Podgorney says: large volumes of deeply injected CO2 should mineralize within a decade or two, long before the gas has a chance to seep out into the atmosphere or into overlying freshwater aquifers.
And the team is now set to take the crucial next step: testing its models with data from experiments around the world. In September, McLing and Podgorney gave invited talks at a CCS conference in Iceland. In addition to munching hors d'oeuvres at the home of Iceland's president, they shared ideas with key members of the CarbFix project. CarbFix — a collaborative effort led by the University of Iceland, Columbia University, Reykjavik Energy and France's National Center for Scientific Research — will be the world's first large-scale test study of mineral sequestration in basalt. CarbFix will dissolve a load of CO2 in water — speeding things up, since it can take a long time for this to happen underground — then inject the seltzer solution about 600 meters (2,000 feet) deep in Icelandic basalt. CarbFix plans to sequester 2,000 tons of carbon dioxide in this manner every year to study mineralization rates and the extent of CO2 leakage. And all of this should start happening within a month or two.
While INL's logo does not yet appear on CarbFix brochures, that may change.
"We'll probably formalize a collaborative relationship soon," McLing says. In any event, the CAES researchers will soon have access to CarbFix data and water samples, which they can use to firm up their models.

Validating the models
"It's your classic murder mystery," McLing says of water sample analysis. "You find the body and try to figure out what happened."
McLing cracks cases by determining what elements and minerals are dissolved in his samples. Water picks up different chemical signatures as it moves from one type of rock to another. Fleshing out these signatures can reveal where the water's been and how quickly certain chemical reactions — such as the ones that turn CO2 into stone — have taken place.
McLing and Podgorney hope to analyze enough samples to figure out definitively how CO2-saturated water moves through basalt. This in turn will help them determine if their carbon mineralization models need tweaking. By early 2010, they should have samples from the CarbFix project in hand — bubbly seltzer from injection areas as well as normal water from control sites, giving the scientists both experimental and baseline data.
By the summer of 2010, the team will also start collecting water samples right from INL's backyard. In September, a major multinational corporation granted McLing and his colleagues $750,000 to conduct a three-year sequestration study at Soda Springs, a small town 60 miles southeast of Idaho Falls. At Soda Springs, naturally carbonated water flows over, under and through basalt very similar to the rock formations found at CarbFix's Iceland site.
"Soda Springs is a natural analogue," McLing says. "We'll collect water from every window we can sample there."
And McLing and Podgorney hope to supplement these samples with water from another site in eastern Washington, where Pacific Northwest National Laboratory plans to lead an injection study similar to CarbFix in the near future.
The CAES scientists are analyzing mineral sequestration from another angle as well. Any day now, a set of 8-vessel pressure systems that the team ordered months ago will be delivered to their lab. This new equipment will allow them to recreate the temperatures and pressures found at potential sequestration depths in basalt. So McLing and Podgorney will have another way to gather data, another tool to test their models and improve their understanding of how mineral sequestration works — and whether it could work on a large scale.
"The point is to get our arms around all of these things, to close the circle," Podgorney says.
Not a silver bullet
Though mineral sequestration shows a great deal of promise, research into the approach is still in its early stages, and many complicating factors remain. Chief among these is cost, a problem for all forms of CCS technology. Scrubbing CO2 from smokestacks, processing it and transporting it to injection sites would chew up about 25 percent of a typical coal-fired plant's energy output, McLing estimates. So for every three new plants built with carbon capture capabilities, a fourth would be needed just to power the CCS process.
This energy cost translates to a high economic cost as well. McLing says it is likely that no form of CCS is practical in the U.S. without a government-imposed price on CO2, likely somewhere between $50 and $100 per ton. StatoilHydro, for example, only began its CCS operations after Norway implemented a CO2 tax of $55 per ton. The company's CO2 injections now save it $55 million every year.
Also, CCS would only work at point sources such as power plants, which are responsible for about half of the world's human-caused CO2 emissions. Carbon dioxide from cars, trucks, boats and planes would still waft into the atmosphere in huge volumes. For these reasons, the U.S. Geological Survey stresses that CCS is a "necessary but insufficient" measure to control atmospheric CO2. Alternative energy development and conservation, among other strategies, will also be required to get the climate-change problem under control.
McLing and Podgorney, however, are focusing on the positive: that capture and sequestration can be a big part of the solution. And they're excited about the future of mineral sequestration.
"There's an awful lot of energy in the field right now," McLing says. "The doors are really beginning to open."
Provided by INL