Scientists Show How Bacteria Move Electrons Across a Membrane
(PhysOrg.com) -- Scientists at the University of East Anglia, Pacific Northwest National Laboratory, and Pennsylvania State University have demonstrated for the first time the mechanism by which some bacteria can transfer electrons across a membrane to the cell exterior, allowing them to "breathe" metals. These iron-respiring bacteria link the cycling of iron and carbon in subsurface and surface sediments and can catalyze the immobilization of subsurface contaminants such as uranium.
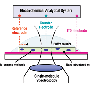
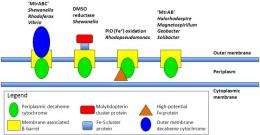
In an article published in Proceedings of the National Academy of Sciences, the researchers described the protein complex -- and its electrochemical properties -- from Shewanella oneidensis, a bacterium renowned for its diverse metabolism and ability to immobilize certain radioactive contaminants. This research demonstrated a novel outer membrane-spanning electron transfer system that enables the proteins MtrA (inward facing) and MtrC (outward facing) to embed sufficiently within a third transmembrane protein, MtrB, to allow electron transfer to take place between them.
"This is an exciting advance in our understanding of bacterial processes in the Earth's subsurfaces," said Dr. David Richardson, of UEA's School of Biological Sciences, who is leading this research effort.
Iron respiration is one of the most ancient respiratory processes for microorganisms. It is among the most common in oxygen-free habitats and therefore has wide environmental significance. In species of Shewanella and other metal-reducing bacteria, some electron transfer proteins, called cytochromes, are positioned at the extracellular face of the bacterium's outer membrane where they can interact with insoluble substrates such as iron oxides. To transfer electrons onto these substrates to gain energy for growth and metabolism, these proteins must be charged by the cell's electron transfer system much of which resides at the inner membrane.
The electron transfer reaction results in an oxidation state change (reduction) in electron acceptors such as iron minerals that are external to the cell. The electron delivery to these proteins requires that electrons originating from the inner membrane be transferred across the outer membrane. The biochemical basis for this has not previously been understood.
The research team used proteoliposomes—lipid vesicles containing proteins—and the redox dye methyl viologen to demonstrate electron transfer across a membrane. They then used protein film voltammetry to measure the electrochemical properties of the proteins, individually and as a complex.
"We discovered that the bacteria can construct tiny biological wires that extend through the cell walls and allow the organism to directly contact, and conduct electrons to, a mineral," said Richardson. "This means that the bacteria can release electrical charge originating from inside the cell to materials such as iron minerals, much like the ground wire on a household plug."
According to the PNNL team, this is the first demonstration of protein complex that catalyzes such an important reaction. The authors also noted that similar MtrA-MtrB modules are present in many other types of bacteria, possibly for different reactions that require electron transfer to, or possibly from, the cell surface.
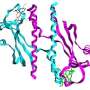
The next steps in this research involve obtaining high-resolution structures for each of the proteins as well as the entire complex to use the structural information in concert with protein interaction data to establish precisely how electrons enter, traverse, and then exit the protein complex. The discovery and functional understanding of protein modules capable of electron transfer across membranes may also be of use in synthetic biology; for example, in bioenergy applications.
More information: Hartshorne RS, CL Reardon, DE Ross, J Nuester, TA Clarke, AJ Gates, PC Mills, JK Fredrickson, JM Zachara, L Shi, AS Beliaev, MJ Marshall, M Tien, SL Brantley, JN Butt, and D Richardson. 2009. "Characterization of an electron conduit between bacteria and the extracellular environment." Proceedings of the National Academy of Sciences of the United States of America 106 (52):22169-22174.
Provided by Pacific Northwest National Laboratory