TWEAK triggers atrophy of disused muscle
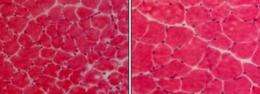
A new study in the Journal of Cell Biology (JCB) identifies a cytokine signaling pathway that induces the breakdown of disused skeletal muscle. Blocking this pathway could prevent immobilized patients from losing their muscle tissue.
Skeletal muscle wastes away when its activity is reduced by, for example, a spinal cord injury. Although the mechanism by which muscle fibers break down is understood fairly well, how the process is triggered remains unknown. The TNF-related cytokine TWEAK can induce muscle loss, but whether it does so in disused muscle is unclear.
A team led by Ashok Kumar (University of Louisville School of Medicine, Kentucky) compared how mice expressing different amounts of TWEAK responded when the nerve innervating their hind legs was severed. Mice producing excess TWEAK lost their muscle more quickly than wild-type animals, whereas mice lacking this cytokine were largely protected from muscle breakdown. TWEAK levels also correlated with the amount of fibrosis, another common symptom of muscle disuse. Inhibiting TWEAK with a neutralizing antibody was sufficient to block muscle breakdown following the loss of motor neurons, suggesting that the pathway could be a viable therapeutic target.
The article appears in the March 22 issue of the Journal of Cell Biology.
More information: Mittal, A., et al. 2010. J. Cell Biol. doi:10.1083/jcb.200909117















