Gene links neurodegeneration and cancer
(PhysOrg.com) -- In work that could lead to new insights into how neurons protect against neurodegeneration, researchers at MIT’s Picower Institute for Learning and Memory report that a gene family known for its role in controlling cell proliferation and suppressing tumors is also essential in the brain to regulate neuronal structure and function.
Understanding how these processes normally work is key to dissecting what goes wrong in a host of neurological and psychiatric diseases. Defects in neuronal wiring have been implicated in developmental disorders such as autism and mental retardation. Working out the mechanisms by which neurons normally control their shapes can provide key insights into how this mechanism goes awry in disease states.
Using the common fruit fly Drosophila as a model, Picower Institute researcher J. Troy Littleton studies the connections that link together neurons within the brain. His lab elucidates how neurons form synaptic connections, how those connections work to transmit information and how the connections change during learning and memory.
“What got us excited about this gene and its protein was the fact that members of this gene family had been previously shown to function as tumor suppressors,” said Littleton, associate professor in the departments of Biology and Brain and Cognitive Sciences at MIT. “This work demonstrates an exciting tie-in of a known cancer-related gene family with a new role in neurodegeneration, potentially linking abnormal regulation of the cell cytoskeleton in pathologies as diverse as cancer and neurological diseases.”
Understanding how a cell’s internal scaffold, or cytoskeleton, mediates these diverse cell processes will shed new light into potential therapeutic approaches to potentially correct defects underlying certain disorders, he said.
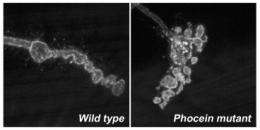
The work revolves around a new member of the Mob protein family known for its role in cell division and as a cancer suppressor. Littleton and Joost Schulte, a postdoctoral associate in the Littleton laboratory, identified a member of this family, a protein called phocein/Mob4, as a key regulator of neuronal structure. When the protein is missing, neurons have abnormal branching patterns and connect improperly to target cells. These defects are tied to disruptions in the cell cytoskeleton. Mutants lacking the protein also have defects in the ability of neurons to transport cargo from the cell body to the synapse, which is critical for neurons to communicate and survive.
“We decided to characterize phocein because it is highly expressed in neurons and was found to control their elaborate branching structure, a key feature that makes neurons unique among other cells found in the body,” Schulte said. Phocein is 80 percent identical between humans and fruit flies, suggesting it is likely to play a similar role across species in regulating neuronal structure.
Neurons extend a single long, thin axon and numerous shorter, thicker, branch-like dendrites. Information gathered and processed by the dendrites flows through the axons to synapses, through which neurons communicate within the brain.
When Schulte knocked out the gene encoding phocein in fruit flies, their neurons become hyperbranched and had defective signaling. Given the role of the Mob family in regulating cell proliferation in cancer, the investigators suspect the protein has similar signaling partners in neurons, controlling the activation of key internal cell regulators.
The researchers stumbled across Phocein by screening the entire Drosophila genome to identify genes required for the formation of normal neural networks. “We were looking for genes that, when knocked out, cause defects in the growth, shape and structure of axons and dendrites,” Littleton said.
By generating Drosophila phocein mutants, the researchers discovered that phocein has key roles in regulating neuronal shape and function within the nervous system.
The researchers are currently working on identifying partners that interact with phocein to better understand how it functions at the molecular level to interface with the underlying cytoskeleton.
More information: “DMob4/Phocein regulates synapse formation, axonal transport and microtubule organization,” Joost Schulte, Katherine Sepp, Ramon Jorquera, Chaohong Wu, Yun Son, Pengyu Hong and J. Troy Littleton in the Journal of Neuroscience, published April 14, 2010.