August 16, 2010 feature
Could thermodynamic fluctuations have led to the origins of life?
In the field of abiogenesis, scientists are currently investigating several ways in which life could have arisen from non-living matter. Generally, any theory of abiogenesis should account for two important aspects of life: replication (the ability to transmit mutations to offspring) and metabolism (the chemical reactions required for vital activities such as breaking down food). Although these two characteristics help to provide a working definition of life, more recently scientists have emphasized the importance of another key feature required for Darwinian evolution: selection, or the replication of mutations that provide an evolutionary advantage.
"The basic problem of abiogenesis is finding the first living entity that generated from non-living matter," Doriano Brogioli, a physicist from the University of Milan-Bicocca, told PhysOrg.com. “But what is the definition of life: is it replication, or metabolism, or simply self-catalysis? I think that it is not simply a matter of definition: what is necessary is 'evolution,' even if the entity that undergoes (or performs) evolution is not a classical living entity. After evolution starts, it can reach whatever complex structures: from a cell, evolution creates trees, whales, birds, ants, and all the prodigious current living world."
Purely chemical systems may possess the ability to replicate and metabolize, but scientists have found that chemical systems by themselves do not undergo selection; more active molecules are not replicated more than others, and useful individual mutations are not inherited by the offspring. Therefore, researchers have suggested that some kind of physical process is likely required to introduce competition among chemical systems and generate the selective pressure required for evolution.
"Three features are needed for evolution: inheritance, mutation and selection," Brogioli said. "Single molecules can replicate other molecules, including other copies of themselves, and can undergo mutations. But in a solution with replicases, each replicase replicates whatever it finds, including non-active molecules or less active replicases. Selection is not active. The reason is that (traditional) chemistry favors selfish molecules: the molecule that is more able to be replicated increases its concentration. In order for selection to take place, there must be a physical process. Confination due to membranes is the current way used by living organisms. But it is hard to believe that a complex structure like a cell can form spontaneously, since the replicating polymers must form together with the membranes themselves. This problem is found in all the theories of abiogenesis, including the RNA-world and the metabolism-first theories."
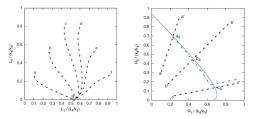
Brogioli has taken a unique approach to satisfying the requirement for selection by proposing that the answer may lie in thermodynamic fluctuations. These fluctuations, which are changes in the number of molecules in a given volume due to thermal motions, may allow selection to become effective, leading to the increase in molecules having an evolutionary advantage. By investigating some chemical systems that possess a feature that he calls "chemical marginal stability," Brogioli has shown that thermodynamic fluctuations induce not only a random walk, but a drift directed toward increasing replication efficiency.
Brogioli suggests that this drift represents an early form of evolution that took place before membranes began to enclose chemical systems; after this time, the membranes would have assumed responsibility for defining entities in competition with one another, allowing selection to take place. If thermodynamic fluctuations did play the role of selection in early life, it would overcome the problem of requiring replicating chemicals and the membranes that enclose them to emerge simultaneously.
In his paper, Brogioli looks at replication from a kinetics perspective, in which the kinetic counterpart of the inheritance of mutations is the presence of multiple stationary states, i.e., different lines of mutations can be present simultaneously, and their offspring inherit mutations. He shows that chemical systems that can pass mutations on to offspring can be thought of as systems with multiple stationary states, thus having the property of chemical marginal stability. These systems differ from a simple "self-catalytic" system (e.g., a purely chemical system) that merely produces offspring without transmitting mutations; the kinetic counterpart would be initial states that all lead to only one stationary state.
As an analogy of a marginally stable system, Brogioli considers the mechanical example of a marble on a flat surface, where any point on the surface is a stationary point. If the marble is disturbed, it reaches a different stationary point rather than returning to its original position, since there is no restoring force. Likewise, spontaneous concentration fluctuations can enable a chemical system to inherit a variety of mutations from its parent system, and any of these mutations can be considered stable.
Brogioli discovered the drift motion by mathematically studying the thermodynamic fluctuations over time. He found that, if two replicases R1 and R2 are present, the most efficient replicase, say R2, begins increasing in number and becomes dominant. In volumes with a higher concentration of R2, more replications will occur, and a larger fraction of them will be R2. Afterward, an even more efficient replicase can arise due to random mutations, and its concentration will increase, and so on.
At the moment, the drift has been confirmed only by numerical calculations, and must be regarded as still theoretical. Brogioli notes that most chemical systems that have a replicase do not possess chemical marginal stability, and therefore are not affected by thermodynamic fluctuations. However, his study shows that the existence of a chemical system that is marginally stable and can undergo spontaneous evolution is possible. Investigating this theory further could have extremely important revelations. The demonstration of a marginally stable chemical system in the lab would not only be the first experiment in which a chemical system undergoes spontaneous evolution, but also the first in vitro model of a chemical reaction that leads to life.
"Currently, no replicase that can self-sustain its replication has been created, but replication of RNA polymers can be obtained by ligation of short oligonucleotides," Brogioli said. "This is a way [to find a chemical system could have marginal stability]. Another possibility is to create a more abstract system, in which replication is accomplished by an enzyme, and the activity of the enzyme is affected by the presence of one of the replicated polymers. Obviously, this is only a proof of principle of marginal stability and evolutionary drift, but not a realistic reproduction of the origin of life. The most interesting possibility is to consider the reactions proposed by the metabolism-first theories. In those theories, no replicating polymer was involved in abiogenesis, but only small molecules constituting some kind of metabolic network. The target is to find a very simple reaction that can be marginally stable."
More information: Doriano Brogioli. "Marginally Stable Chemical Systems as Precursors of Life." Physical Review Letters 105, 058102 (2010). DOI: 10.1103/PhysRevLett105.058102