A promising target for developing treatments against Parkinson's disease
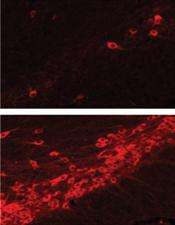
Researchers at Johns Hopkins have shown that using specific drugs can protect nerve cells in mice from the lethal effects of Parkinson's disease. The researchers' findings are published in the August 22 issue of Nature Medicine.
The newly discovered drugs block a protein that, when altered in people, leads to Parkinson's disease. Parkinson's disease causes deterioration of the nervous system that leads to tremors and problems with muscle movement and coordination. There is no proven protective treatment yet. Only recently have genetic causes of Parkinson's disease been identified that have the potential to be used for developing targeted therapies for patients with the disease.
The protein LRRK2 (pronounced lark 2) is overactive in some Parkinson's disease patients and causes nerve cells to shrivel up and die. Why exactly overactive LRRK2 is toxic and leads to Parkinson's disease is still unknown.
Since overactive LRRK2 is deadly, researchers speculated that blocking LRRK2 from acting might protect nerve cells. The research team tested drugs that were commercially available and known to prevent proteins like LRRK2 from acting and adding chemical phosphates to other proteins. Out of 70 drugs tested, eight were found to block LRRK2 from working.
Two of these eight previously were shown by others to be able to cross the blood-brain barrier. So the researchers injected these two drugs twice daily into mice engineered to carry Parkinson-causing LRRK2 changes in their brain. After three weeks, they examined the mouse brains to see if nerve cells had died. One drug provided almost complete protection against nerve cell death. Another drug had about 80 percent fewer dead cells than in mock treated mice. A third drug, which does not inhibit LRRK2 was not effective.
"This data suggests that if you were to develop a safe drug, then you could potentially have a new treatment for Parkinson's disease patients with LRRK2 mutations," says Ted Dawson, M.D., Ph.D., professor of neurology and physiology and scientific director of the Johns Hopkins Institute for Cell Engineering.
The two drugs that blocked LRRK2 and prevented death of nerve cells in mice with Parkinson's disease both had similar chemical structures. "One could envision generating compounds around that core structure to develop a relatively selective and potent inhibitor of LRRK2," says Dawson.
Dawson is collaborating with researchers at Southern Methodist University to design more specific inhibitors of LRRK2 and the group plans to license this technology. Once they identify promising candidate drugs, those candidates still will have to be tested for toxic side effects. The drugs' approval by the FDA for use in humans may still be many years away.
Says Dawson, treatments developed specifically against LRRK2 may even be able to treat other forms of Parkinson's disease — those not caused by LRRK2 alterations — as there may be several alterations in different proteins that can lead Parkinson's disease.
"We're curing Parkinson's disease in a mouse and now we have to discover drugs that actually work in human neurons. Then we'll hopefully be able to make the leap forward to get a treatment to work in humans," says Dawson.















