May 8, 2006 feature
Scientists discover water is sticky on a small scale
When water vapor condenses in a nano-sized space between two surfaces, the liquid behaves more like solid ice than liquid water, even at room temperature. This solidification causes water to exert such a strong friction force that it “acts like a glue,” according to a new study.
If you’ve ever thought about ice skating on a dry floor – or anywhere besides a sheet of ice – you can easily imagine how you’d ruin your blades and not get very far. Ice skating – like many processes involving moving parts – requires water acting as a lubricant between the blades and the solid ice, reducing friction and keeping the sliding surfaces apart from each other.
Now imagine you’re five nanometers tall and want to go ice skating, or that you want to build a nano-sized sliding element using water as a lubricant. Scientists who have recently been experimenting with tiny moving devices have run into problems due to stiction (static friction) forces between moving parts.
“Micro- and nano-electromechanical systems (MEMS and NEMS) have tiny moving parts typically etched out of silicon,” said J. Frenken, coauthor with K. Jinesh of a recent Physical Review Letter, to PhysOrg.com. “Using special tricks such as under-etching, intricate structures can be formed with cogwheels, hinges, bending arms and sliding elements.”
However, mechanical systems on the micro and nano scales endure much greater friction than their macroscopic counterparts.
“Many of the designs for such miniature devices are useless in practice because the parts either don't move at all anymore or they move with very high friction, which leads to excessive wear,” said Frenken. “After a short time, this wear destroys the device. One could state that many MEMS devices work in ‘self-destruct’ mode.”
Jinesh and Frenken explain that the reason for these lube problems is that, on the nanoscale, water ceases to be wet and slippery and instead becomes solid and sticky. By studying the atomic and molecular details of water and the surfaces sandwiching the water, the scientists found condensed water is immediately converted into ice, acting more like a glue than a lubricant. This finding contrasts previous studies, which suggested that water acts like a lubricant down to the nanoscale.
“In many of these MEMS and NEMS structures, there are elements that are either touching each other or close to touching each other,” said Frenken. “At the locations where two objects approach each other within a very small distance, water from the air tends to condense. This capillary condensation strongly pulls the (nearly) touching objects into intimate contact. To avoid friction and wear, these elements need to be lubricated with a liquid that would remain slippery down to the nanoscale – actually, the lubricant should become even more slippery than it is on a macroscopic scale.”
In their experiment, the scientists built a friction force microscope, which consists of a hydrophilic tungsten tip scanning back and forth across a hydrophobic graphite surface. In extremely dry conditions, the tip demonstrated expected “stick-slip motion,” meaning it would generally move evenly, while occasionally slipping ahead at regular intervals.
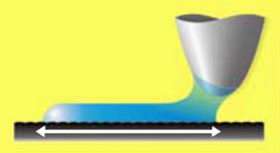
At higher humidities (relative humidity greater than 10%), however, the scientists did not observe this motion due to water vapor condensing in the capillary between the tip and surface. The water exerts an elastic force on the tip, and as the tip moves, the water stays connected with it, appearing to stretch across the surface. (See above image.)
The static stress and regular structure of the condensate makes the scientists conclude that the condensate acts like a solid. The team observed the tip “write” a stripe of water up to 250 nanometers, which lasted for up to two seconds and beyond the point where the tip scans above the condensate.
“Although in many cases, water acting as a glue on the nanoscale is a nuisance, there are also situations where one can take advantage of the stickiness on this small scale,” said Frenken. “On a macroscopic scale, we all know how dental prostheses that can stay in place via the action of bridging water. In this case, however, the water is still completely liquid because of the thickness of the water layers. In the laboratory, we often use capillary condensation to pick up light objects, simply touching a small object with a thin wire, instead of using tweezers. A capillary neck forms between the object and the wire, gluing the two together.” This “nuisance,” then, may lead to more useful applications, in addition to the problem it identified.
Citation: Jinesh, K. B. and Frenken, J. W. M. Capillary Condensation in Atomic Scale Friction: How Water Acts like a Glue. Physical Review Letters. 96, 166103 (2006).
By Lisa Zyga, Copyright 2006 PhysOrg.com