First Molecular Proof That Some Aspects of Aging Are Out of Our Control
Aging -- a random affair? A study by Buck faculty provides first molecular evidence that some aspects of aging are likely out of our control.
There’s no argument that eating well, exercising wisely, and avoiding high risk behaviors can increase one’s chances for a longer, healthier old age. But it’s also obvious that in many ways the aging process is out of our control; that despite our best efforts (in concert with a genetic make-up that makes us more or less susceptible to certain diseases) our cells and tissues ultimately degenerate and eventually die. While scientists have long suspected that events outside our control can result in aging, a study led by Buck Institute faculty member Jan Vijg, PhD, provides the first direct evidence that the molecular machinery of our cells providing function to our tissues and organs spins irreversibly out of control as we age. The study appears in the June 22 edition of Nature.
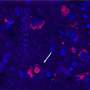
The study identified and measured increases in random deviations in the activity of genes in aging mouse hearts. The work, done when Vijg was at the University of Texas Health Sciences Center in San Antonio, compared single cells from cardiac tissue in both young and old mice that were genetically identical, bred from the same genetic strain. Scientists focused on the activity of sets of arbitrarily chosen genes in individual cardiomyocytes taken from the young and old mice. While at a young age, the different cells displayed very similar activity levels for each gene. At old age, however, they were out of tune, expressing a given gene at sometimes greatly different levels, which is likely to adversely affect the instructions to proteins involved in cardiac tissue function. This is the first time that scientists were able to identify, measure and quantify an increase in gene activity levels in the aged mouse cells, possibly pointing to a random breakdown of gene regulation.
“In younger hearts the cells beat in tune, working together to carry out the functions associated with cardiac tissue,” said Vijg. “As the cells age, they start to diverge, and no longer have the same activity levels. Now that we have a means of identifying and measuring these cell-to-cell variations in gene activity, we can begin to focus on how they cause tissues and organs to become dysfunctional over time,” said Vijg.
What is the cause of such an increase in dissimilar gene activity among cells supposed to act in a very similar way? One possibility is that a gradual accumulation of random DNA alterations, also termed “mutations” begin to interfere with transcription, i.e., the process that generates the message from each gene that contains the instructions to make a protein. Such interference can be a result of mutations that cause, for example, a loss of functional gene copies or the inactivation of DNA stretches that serve to control gene activity. To test this possibility, Vijg and his team treated mouse embryo cells with hydrogen peroxide, a free radical-generating agent that damages DNA. In a sense this treatment induces a form of “artificial” aging. The hydrogen peroxide treatment resulted in a significant increase in cell-to-cell variation in gene expression in the embryonic tissue, paralleling the increase found in the cardiac tissue of the older mice.
Vijg, who is internationally known for his groundbreaking work on genomic instability in aging and cancer, said it has been impossible thus far to link the accumulation of random genetic damage to functional consequences in aging organisms, with the exception of cancer, which is known to be caused by DNA mutations. This may offer a possible mechanism through which mutation accumulation can cause cell and tissue dysfunction during aging. The work underscores the random nature of the aging process, which also manifests at the level of the whole organism. “Genetically, twins are nearly identical when they are young,” said Vijg. “As they age, their genetic differences become more pronounced. While some differences can likely be attributed to environmental influences, there is little doubt that aging itself is something of a random affair. Indeed, recent work by others provided evidence that the divergence of identical twins also involves alterations in the genome.”
“Jan’s novel approaches to the study of aging continue to provide important insights into this process,” said Dale Bredesen MD, Buck Institute CEO and Scientific Director. “His new finding that aging tends not only to dysfunction, but also to a loss of uniformity gives us another new view. We’re excited he and his team have joined the Buck Institute,” said Bredesen.
Those joining Vijg in the study included Rumana Bahar, Rita Busuttil, and R. Brent Calder, who also came to the Buck Institute from Texas. Other contributors include Karl Rodriguez, Ashley Denny, Gary Chisholm, and Brad Pollock, from the University of Texas Health Science Center; Claudia Hartmann and Christoph Klein from the Institute for Immunology, Ludwig-Maximilians University in Munchen, Germany; and Martijn E. T. Dolle, from the National Institute of Public Health and the Environment, Bilthoven, the Netherlands. The work was supported by a grant from the National Institutes of Health and a BioFuture Grant from the German Federal Ministry for Education and Science.
Source: Buck Institute for Age Research