Scientists solve mystery of how largest cellular motor protein powers movement
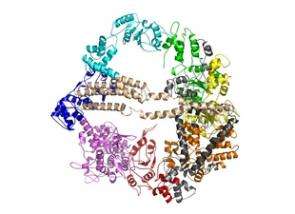
Scientists now understand how an important protein converts chemical energy to mechanical force, thus powering the process of cell division, thanks to a new structural model by University of North Carolina at Chapel Hill researchers.
The structural model helps solve a scientific mystery: how the protein dynein fuels itself to perform cellular functions vital to life. These functions include mitosis, or cell division into identical cells.
Dynein uses energy derived from ATP, or adenosine triphosphate, a molecule that is the principal form of energy for cells. The lack of a comprehensive and detailed molecular structure for dynein has kept scientists largely in the dark about how the protein converts ATP into mechanical force, said Dr. Nikolay V. Dokholyan, assistant professor of biochemistry and biophysics in the UNC School of Medicine.
Dokholyan said the dynein puzzle is similar to figuring out how auto engines make cars move. “You have an engine up front that burns gas, but we didn’t know how the wheels are made to move.”
Dr. Timothy Elston, associate professor of pharmacology and director of the School of Medicine’s bioinformatics and computational biology program, explains further. “One of the unknowns about dynein was that the molecular site where chemical energy is initially released from ATP is very far away from where the mechanical force occurs. The mechanical force must be transmitted over a large distance.”
The study was published online Nov. 22 in the Proceedings of the National Academy of Sciences Early Edition. The work was supported in part by grants from the Muscular Dystrophy Association and the American Heart Association.
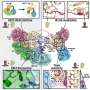
Using a variety of modeling techniques that allowed resolution at the level of atoms, Adrian W.R. Serohijos, a graduate student in Dokholyan’s lab and first author of the study, identified a flexible, spring-like “coiled-coil” region within dynein. It couples the motor protein to the distant ATP site.
“This dynein coiled-coil was completely missing from all previous studies. We saw it could allow a very rapid transduction of chemical energy into mechanical energy,” Dokholyan said.
Conversion to mechanical energy allows dynein to transport cellular structures such as mitochondria that perform specific jobs such as energy generation, protein production and cell maintenance. Dynein also helps force apart chromosomes during cell division.
“Dividing cells must separate their chromosomes and something has to generate the force to move chromosomes apart. Dynein provides the mechanical energy to do that,” Doholyan said.
While the research offers no immediate application to human disease, the authors noted that mutations of dynein have been implicated in some neurodegenerative and kidney disorders. Dokholyan pointed out that disruption of dynein’s interaction with a particular regulator protein causes defects in nerve cell transmission and mimics the symptoms of people with amyotrophic lateral sclerosis (ALS).
Source: University of North Carolina School of Medicine