Spelling out cancer on the nanoscale
Tumors start small and stay quiet, yet their intentions are clearly spelled out internally, if we could only read them. No matter how small, every tumor reveals its identity in tiny amounts of abnormally expressed proteins called oncoproteins. Being able to read trace oncoprotein levels from early-stage tumors would speak volumes to physicians and cancer researchers. Enter nano-fluidics and the art of reading a lot from very little.
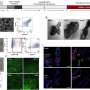
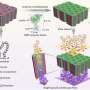
Researchers led by Dean Felsher at Stanford University School of Medicine, and bioengineers at Cell Biosciences in Palo Alto, collaborated to develop an automated, high-throughput, nano-fluidic system that was able to measure the levels of three oncoproteins: MYC, BCL2, and AKT, in tiny samples drawn as very fine needle aspirates from hematopoetic tumor cells in preclinical transgenic mouse models. The nano-fluidic system physically separates the proteins in very small capillary tubes and then uses antibodies for protein detection. It was also tested on human lymphoma samples.
In previous work, the Felsher group had shown that inactivating MYC induces sustained tumor regression in mice. The researchers decided to test their conditional model of MYC-induced lymphoma by using their nano-fluidic system to quickly measure the impact of targeted protein inhibitors in mice. A series of fine needle-aspirated samples was analyzed, confirming a decrease in oncoprotein levels.
The new nano-fluidic system was also tested on human tumors by measuring the levels of MYC, BCL2, ERK, and AKT proteins in the lymph nodes obtained from patients with Burkitt’s, mantle cell, or follicular lymphoma. The nano-fluidic system reported that MYC was overexpressed in Burkitt’s, while BCL2 was overexpressed in mantle cell and follicular lymphoma patients. In parallel, traditional Western blots were performed to confirm BCL-2 and MYC levels.
"Our strategy can be used to repetitively and quickly assess the levels of oncoproteins in cancer cells grown in the laboratory and in human patients," says Felsher. "It may prove useful for the early detection of cancer, and for monitoring patients’ responses during their treatment, allowing clinicians to tailor treatments to individual patients. Finally, we provide a high throughput method to identify if new cancer drugs are effective at targeting specific oncoproteins."
Source: American Society for Cell Biology