Quick Nanocontainer Construction
Nanoscale containers are in high demand because of their ability to enclose molecules. They can, for example, be used as molecular reaction chambers for controlled chemical reactions or as transporters and storage containers for drugs or pesticides; they can also filter poisonous substances out of water. The synthesis of such capsules is not easy.
American researchers at Rutgers University in New Jersey have now produced an octahedral nanocontainer whose 18 components can be assembled in a single, elegant reaction step.
The chemical linking of individual components into a molecular container big enough to hold multiple or large guest molecules is usually an extremely fiddly undertaking, involving many complicated reaction steps. Therefore, a different alternative has been used until now: individual components with the ability to organize themselves into a supermolecular structure by a self-assembly process. In this process the components are not held together by solid chemical (covalent) bonds; instead, they are an aggregate of individual molecules. A team headed by Ralf Warmuth has now found a way to assemble the individual components into a single, giant, container-shaped molecule just as smoothly and spontaneously.
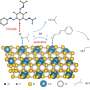
For their synthesis, the researchers used two different “building blocks”: six large, bowl-shaped molecules and twelve small molecules to act as bridging elements. Each bowl has four groups of atoms that particularly like to form links with the reactive amino groups at the ends of the bridges. The two substances need only be dissolved and mixed together in the right proportions to react, to form octahedral capsule-like macromolecules.
The success of this small miracle depends on the flawless formation of an astounding 24 new imine bonds. How does this work? There are two secrets: The octahedral arrangement is the most energetically favorable of the possible forms, and the formation of the bonds is reversible, meaning that the freshly formed imine bonds can easily come apart again under the reaction conditions used. The components thus have the opportunity to arrange themselves into the preferred configuration little by little.
“The octahedral containers make it possible to introduce variations, which should make it possible to produce targeted, tailored containers,” says Warmuth. “We are currently testing to see if our synthetic principle can also be applied to other molecular building blocks.”
Author: Ralf Warmuth, Rutgers University, Piscataway (USA), rutchem.rutgers.edu/content_dy … y/ralf_warmuth.shtml
One-Pot, 18-Component Synthesis of an Octahedral Nanocontainer Molecule
Angewandte Chemie International Edition, doi: 10.1002/anie.200504049
Source: Angewandte Chemie